Question: Use your results and observations in Data Tables 1 , 2 , and 3 , to create a flow chart for the identification of an
Use your results and observations in Data Tables 1, 2, and 3, to create a flow chart for the identification of an unknown (both an unknown anion and cation), using the AgNO3 and HCl confirmation tests and the cation flame tests.
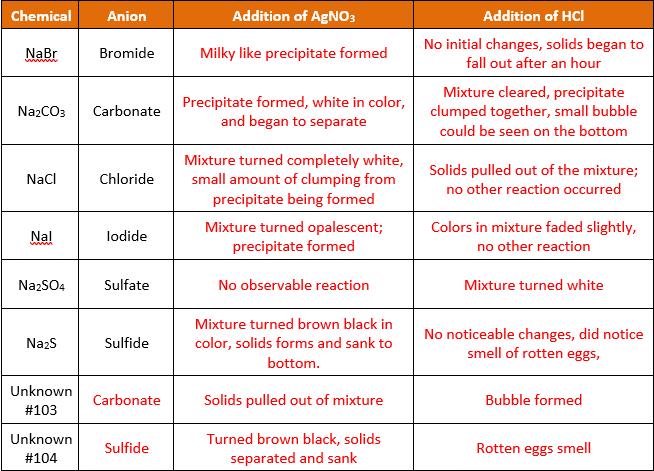
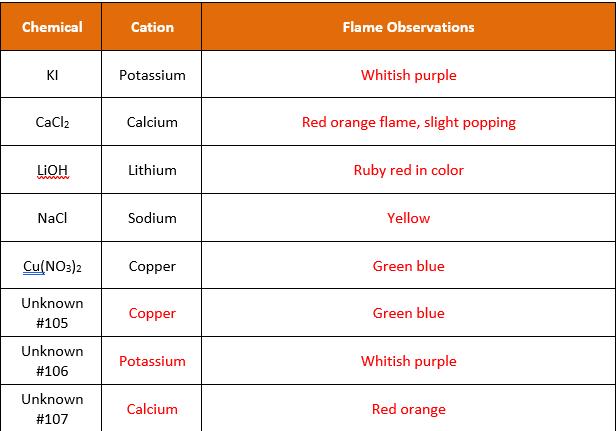
Chemical Anion Addition of AGNO3 Addition of HCI No initial changes, solids began to NaBr Bromide Milky like precipitate formed fall out after an hour Precipitate formed, white in color, and began to separate Mixture cleared, precipitate clumped together, small bubble Na2CO3 Carbonate could be seen on the bottom Mixture turned completely white, small amount of clumping from precipitate being formed Solids pulled out of the mixture; no other reaction occurred Naci Chloride Mixture turned opalescent; precipitate formed Colors in mixture faded slightly, Nal lodide no other reaction NazSO4 Sulfate No observable reaction Mixture turned white Mixture turned brown black in No noticeable changes, did notice smell of rotten eggs, Nazs Sulfide color, solids forms and sank to bottom. Unknown Carbonate Solids pulled out of mixture Bubble formed #103 Unknown Turned brown black, solids Sulfide Rotten eggs smell #104 separated and sank
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it
Unknown 105 Copper Nitrate cation Copper it gives green blue colur to the flame Anion Nitrate No rea... View full answer

Get step-by-step solutions from verified subject matter experts



