Question: 3- Five kg of butane (C,H) in a piston-cylinder assembly undergo a process from p, = 5 MPa, T1 = 500 K to p2

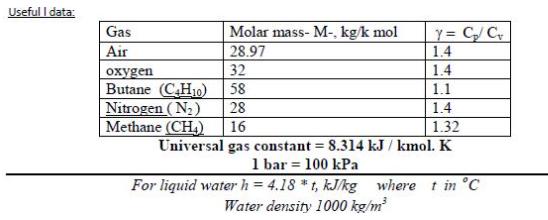
3- Five kg of butane (C,H) in a piston-cylinder assembly undergo a process from p, = 5 MPa, T1 = 500 K to p2 = 3 MPa, T =450 K during which the relationship between pressure and specific volume is pv" = constant. Determine the work, in kJ. Useful I data: Y = C/C 1.4 1.4 Gas Molar mass- M-, kg/k mol 28.97 Air 32 oxygen Butane (CH10) Nitrogen (N2) Methane (CH) 58 1.1 28 1.4 16 1.32 Universal gas constant = 8.314 kJ / kmol. K 1 bar = 100 kPa For liquid water h = 4.18 * t, kJ/kg where t in C Water density 1000 kg/m
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
To determine the work done during the process we can use the relationship between pressure and speci... View full answer

Get step-by-step solutions from verified subject matter experts


