Question: Using an enthalpy cycle, estimate rH. for the decomposition of K2O2 to K2O. The size of the ions are as follows, O is 140pm,Kis138pm, and
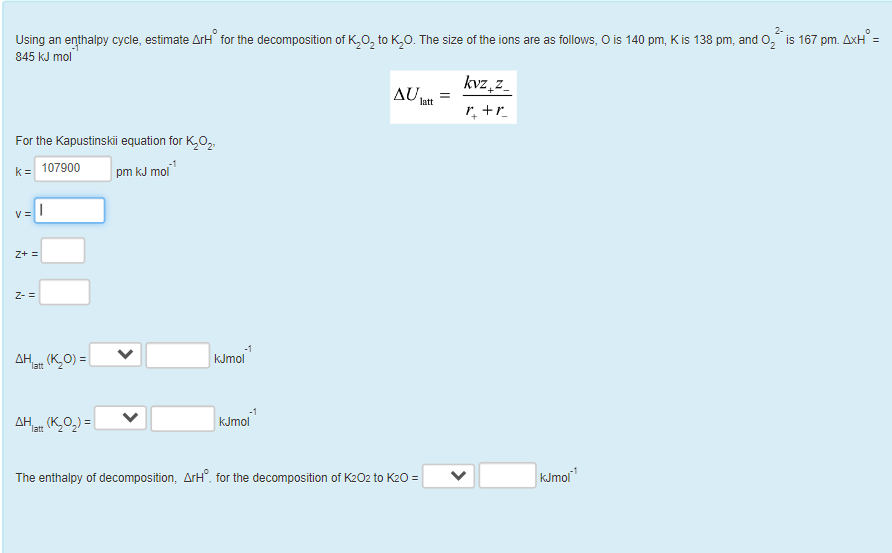
Using an enthalpy cycle, estimate rH. for the decomposition of K2O2 to K2O. The size of the ions are as follows, O is 140pm,Kis138pm, and O22 is 167pm. H= 845kJmol1 Ulatt=r++rkvz+z For the Kapustinskii equation for K2O2, k=v=z+=z= Hlatt(K2O)=kJmol Hlat(K2O2)=kJmol The enthalpy of decomposition, rH. for the decomposition of K2O2 to K2O= kJmol1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


