Question: Using data below and other data in your text if necessary, calculate the enthalpy change for the reaction: Fe2O3(s)+3C(s, graphite) 2Fe(s)+3CO(g) Hf of Fe2O3(s)=824.2kJ/molHf of
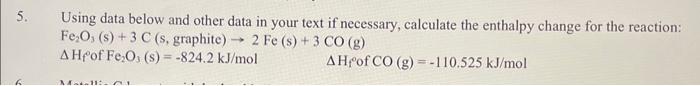
Using data below and other data in your text if necessary, calculate the enthalpy change for the reaction: Fe2O3(s)+3C(s, graphite) 2Fe(s)+3CO(g) Hf of Fe2O3(s)=824.2kJ/molHf of CO(g)=110.525kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


