Question: Using data from trial 4,5,7 calculate ka and percent ionization for ethanoic acid. Standard Used (sodium hydroxide) (base) Name Volume mL burette burette reading (start)
Using data from trial 4,5,7 calculate ka and percent ionization for ethanoic acid.
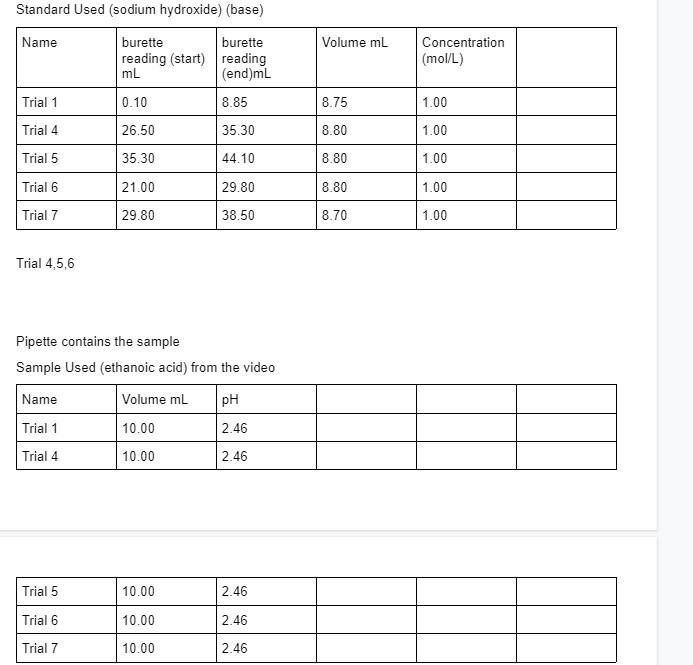

Standard Used (sodium hydroxide) (base) Name Volume mL burette burette reading (start) reading mL (end)ml Concentration (mol/L) Trial 1 0.10 8.85 8.75 1.00 Trial 4 26.50 35.30 8.80 1.00 Trial 5 35.30 44.10 8.80 1.00 Trial 6 21.00 29.80 8.80 1.00 Trial 7 29.80 38.50 8.70 1.00 Trial 4,5,6 Pipette contains the sample Sample Used (ethanoic acid) from the video Name Volume ml pH Trial 1 10.00 2.46 Trial 4 10.00 2.46 Trial 5 10.00 2.46 Trial 6 10.00 2.46 Trial 7 10.00 2.46 Analysis: Calculate the K and percent ionization for the ethanoic acid sample. Show as much work, as clearly as possible. Hand-write your calculations. Include correct units of measurement and significant digits as appropriate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


