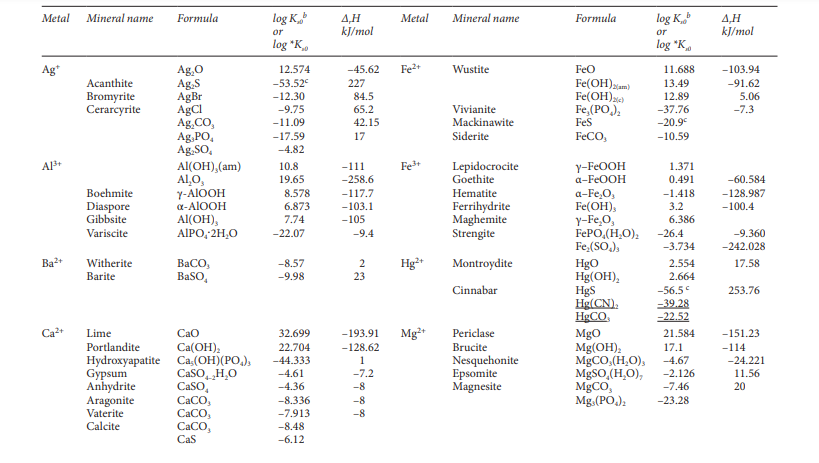
Question: Using Ksp values from Table , show why Ca is removed as CaCO 3 and Mg is removed as Mg ( OH ) 2 during
Using Ksp values from Table show why Ca is removed as CaCO and Mg is
removed as MgOH during softening. Assume a typical TOTCO of M
for your calculations
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


