Question: Using temperature data from the experimental runs a graph is created plotting Ink versus 1/T where T is in Kelvin. The best fit line for
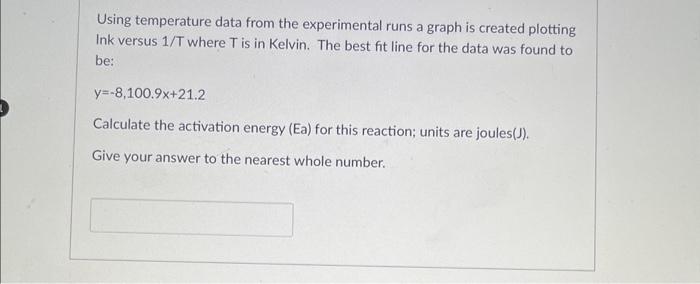
Using temperature data from the experimental runs a graph is created plotting Ink versus 1/T where T is in Kelvin. The best fit line for the data was found to be: y=8,100.9x+21.2 Calculate the activation energy (Ea) for this reaction; units are joules(J). Give your answer to the nearest whole number
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


