Question: Using the approach described in Chapter 6 (along with Table 6.4), (a) write a balanced overall reaction for the oxidation of methanol to CO2 coupled
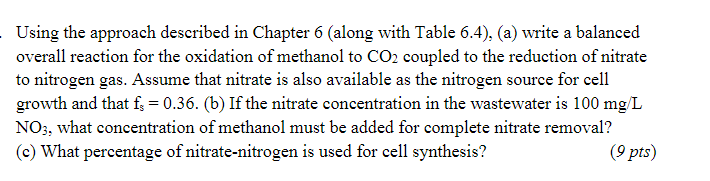
Using the approach described in Chapter 6 (along with Table 6.4), (a) write a balanced overall reaction for the oxidation of methanol to CO2 coupled to the reduction of nitrate to nitrogen gas. Assume that nitrate is also available as the nitrogen source for cell growth and that fs = 0.36. (b) If the nitrate concentration in the wastewater is 100 mg/L NO3, what concentration of methanol must be added for complete nitrate removal? (c) What percentage of nitrate-nitrogen is used for cell synthesis? (9 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


