Question: Using the constance above, answer the question Joule (J) Calorie (cal) Avogadro's number N Coulomb (C) Electron charge Kelvin temperature scale (K) Gas constant
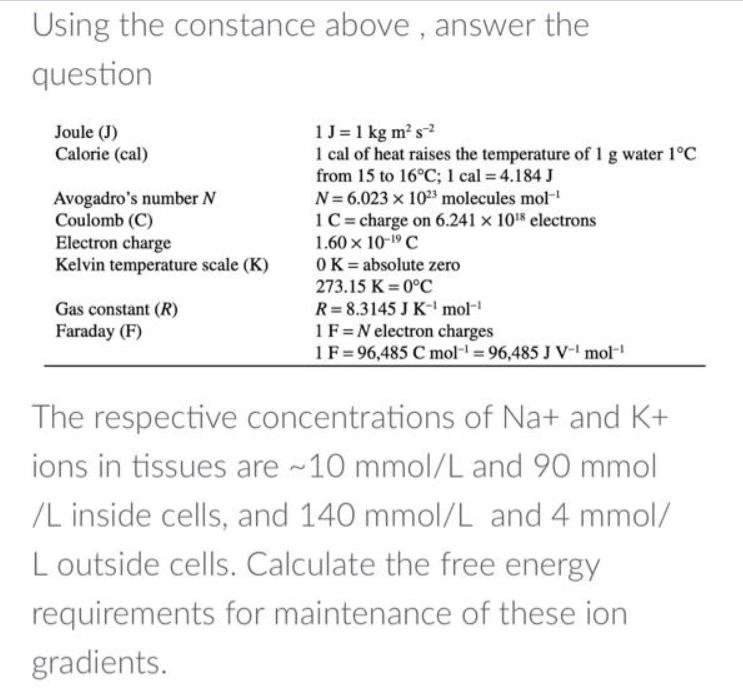
Using the constance above, answer the question Joule (J) Calorie (cal) Avogadro's number N Coulomb (C) Electron charge Kelvin temperature scale (K) Gas constant (R) Faraday (F) 1 J = 1 kg m s 1 cal of heat raises the temperature of 1 g water 1C from 15 to 16C; 1 cal = 4.184 J N=6.023 x 1023 molecules mol-' 1C charge on 6.241 x 1018 electrons 1.60 x 10-19 C OK = absolute zero 273.15 K=0C R=8.3145 J K mol- 1 F N electron charges 1 F 96,485 C mol-1-96,485 J V-1 mol-' The respective concentrations of Na+ and K+ ions in tissues are ~10 mmol/L and 90 mmol /L inside cells, and 140 mmol/L and 4 mmol/ Loutside cells. Calculate the free energy requirements for maintenance of these ion gradients.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


