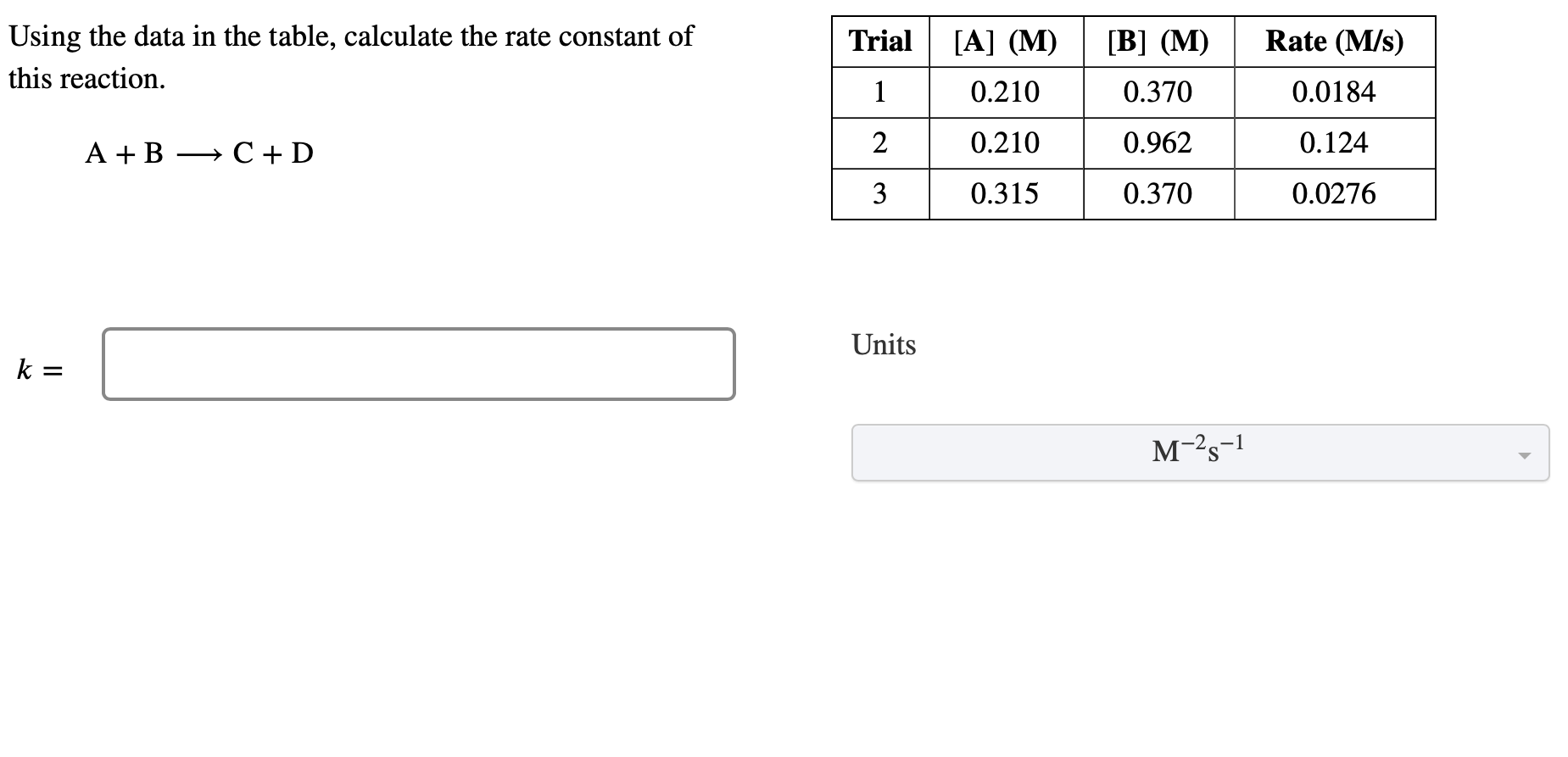
Question: Using the data in the table, calculate the rate constant of this reaction. A+BC+D Trial [] () [] () Rate (M/s) 1 0.210 0.370 0.0184
Using the data in the table, calculate the rate constant of this reaction.
A+BC+D
| Trial | [] () | [] () | Rate (M/s) |
|---|---|---|---|
| 1 | 0.210 | 0.370 | 0.0184 |
| 2 | 0.210 | 0.962 | 0.124 |
| 3 | 0.315 | 0.370 | 0.0276 |
Using the data in the table, calculate the rate constant of this reaction. A+BC+D k= Units M2s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


