Question: USING THE DATA PROVIDED, IF THE QUESTIONS COULD BE ANSWERED AND WHERE MATH IS INVOLVED, IF THE WORK CAN BE SHOWN. WILL RATE THUMBS UP
USING THE DATA PROVIDED, IF THE QUESTIONS COULD BE ANSWERED AND WHERE MATH IS INVOLVED, IF THE WORK CAN BE SHOWN.
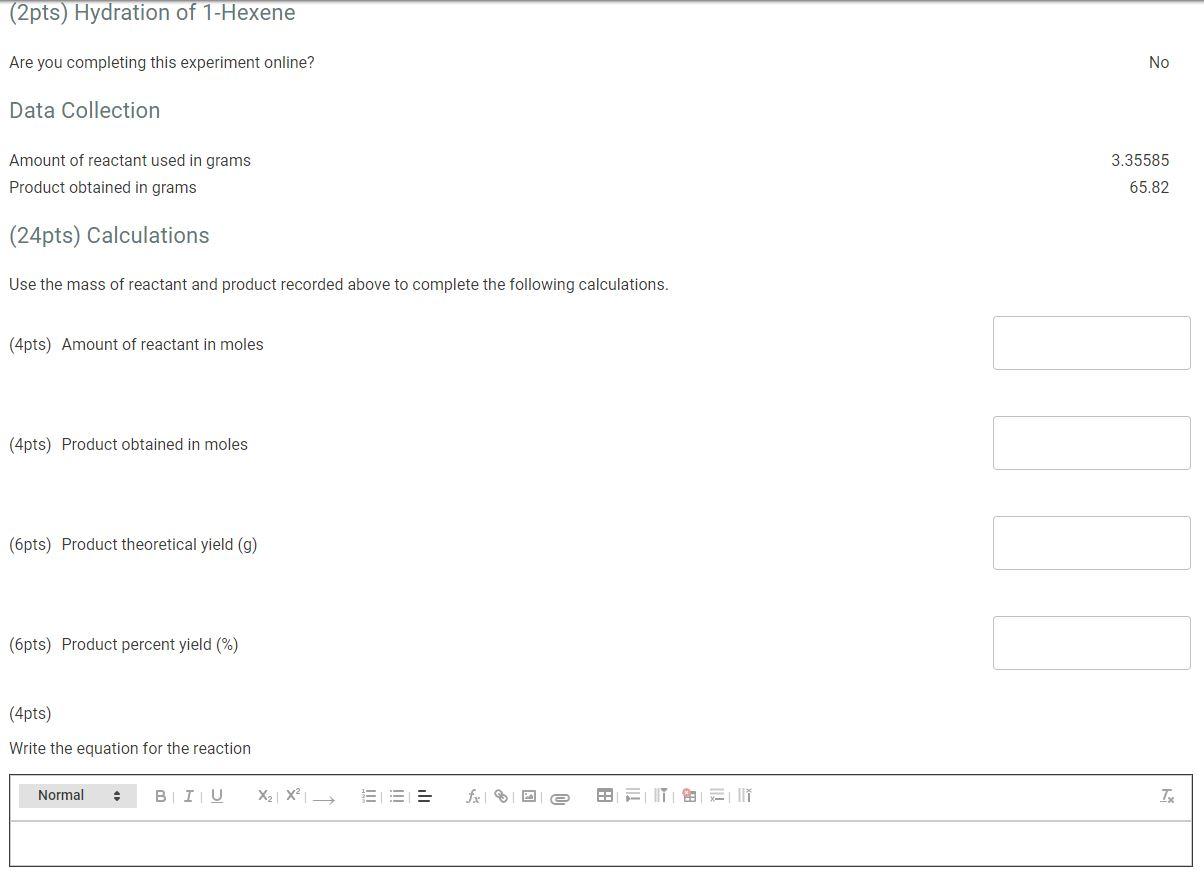
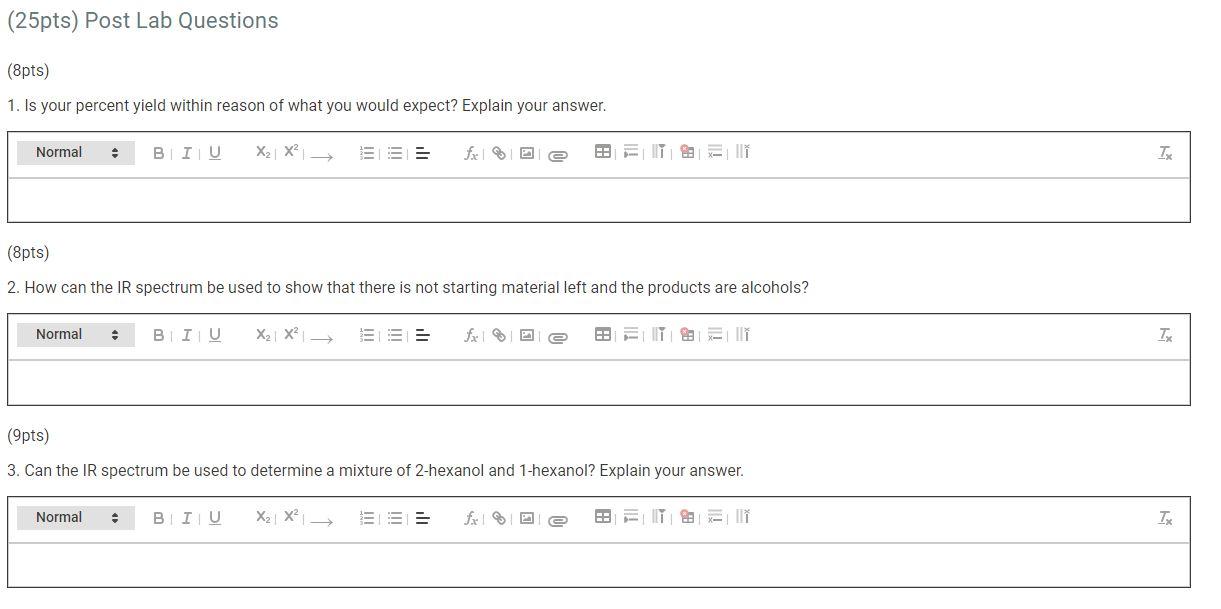
WILL RATE THUMBS UP (2pts) Hydration of 1-Hexene Are you completing this experiment online? No Data Collection Amount of reactant used in grams Product obtained in grams 3.35585 65.82 (24pts) Calculations Use the mass of reactant and product recorded above to complete the following calculations. (4pts) Amount of reactant in moles (4pts) Product obtained in moles (6 pts) Product theoretical yield (9) (6pts) Product percent yield (%) (4pts) Write the equation for the reaction Normal . BIU X2 X = IT891 TE (25pts) Post Lab Questions (8pts) 1. Is your percent yield within reason of what you would expect? Explain your answer. Normal BIU X2 X == fx e FH IT: all in Tx (8pts) 2. How can the IR spectrum be used to show that there is not starting material left and the products are alcohols? Normal BIU X2 X2 = Sex ea ill TK (9pts) 3. Can the IR spectrum be used to determine a mixture of 2-hexanol and 1-hexanol? Explain your answer. Normal BIU X2 X free | xi TX
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


