Question: Using the differential method, for the reaction A products determine the reaction order and the corresponding rate constant for the reaction. The following experimental data
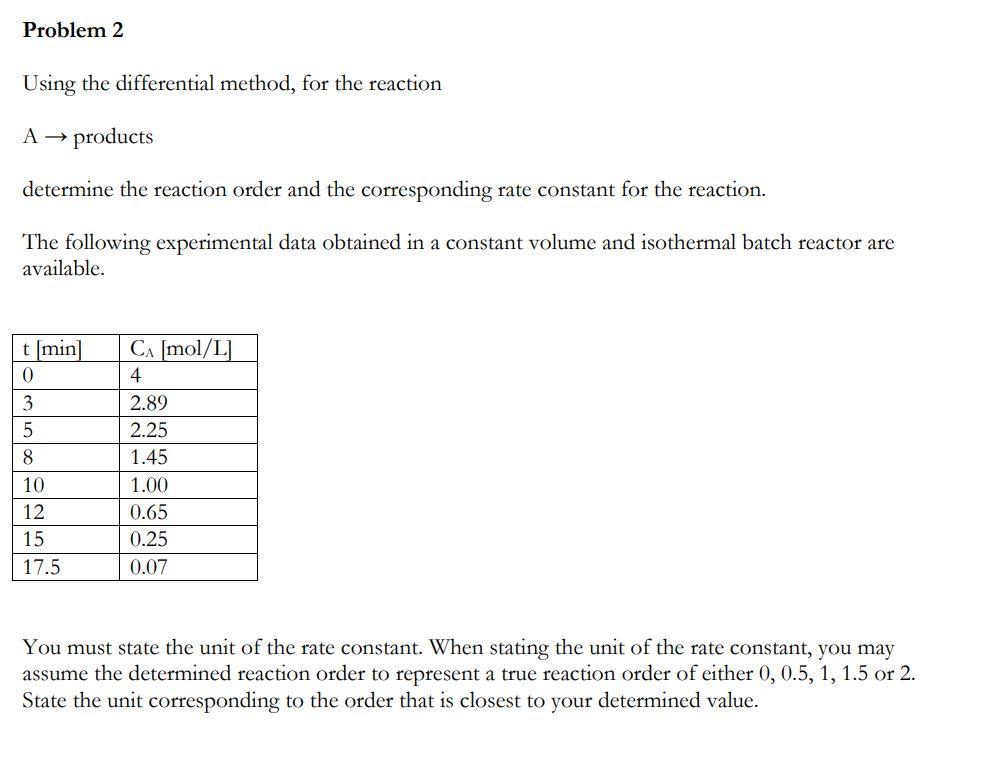
Using the differential method, for the reaction A products determine the reaction order and the corresponding rate constant for the reaction. The following experimental data obtained in a constant volume and isothermal batch reactor are available. You must state the unit of the rate constant. When stating the unit of the rate constant, you may assume the determined reaction order to represent a true reaction order of either 0,0.5,1,1.5 or 2 . State the unit corresponding to the order that is closest to your determined value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


