Question: Using the empirical formula for the unknown solute, , and the group average, determine the molecular formula for the unknown solute. Group average = 153.02
Using the empirical formula for the unknown solute,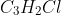 , and the group average, determine the molecular formula for the unknown solute.
, and the group average, determine the molecular formula for the unknown solute.
Group average = 153.02
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


