Question: Using the equation below: a. Balance the reaction by using the half reaction method. b. Calculate the cell potential expected. c. State whether the reaction

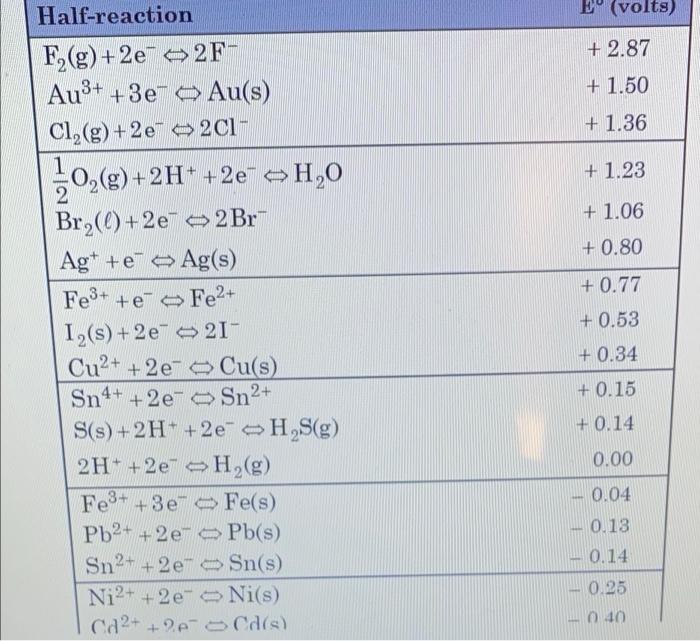
Using the equation below: a. Balance the reaction by using the half reaction method. b. Calculate the cell potential expected. c. State whether the reaction is spontaneous or not. MnO4 + H+ + Cl Mn2+ + Cl2() +H2O (Note: the electron potential for the MnO4 reduction half reaction is +1.52 V, the Cl2 half reaction can be found on your data table.) E (volts) + 2.87 + 1.50 + 1.36 10,()+2H+ +2e" ~H,0 + 1.23 + 1.06 + 0.80 + 0.77 Half-reaction F2(g) +2e2F- Au3+ +3e- Au(s) Cl2(g) +2e2C1- 1 g 2 Br2(0)+2e2Br Ag+ + eAg(s) Fe3+ +e-Fe2+ 1,(s) +2 e 21 Cu2+ + 2e Cu(s) Sn4+ + 2e- Sn2+ S(s) + 2H+ +2e H S(g) 2H+ +2e H2(g) Fe3+ + 3e Fe(s) Pb2+ + 2e Pb(s) Sn2+ + 2e- Sn(s) Ni2+ + 2e- Ni(s) Ca2+ + 2-5 Cd(s) + 0.53 + 0.34 + 0.15 + 0.14 0.00 -0.04 0.18 0.14 0.25 010
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


