Question: Using the equilibrium constant expression given below, calculate the equilibrium constant if [N2]=0.075M,[H2]=7.6103M, and [NH3]=7.9104M. Keq=[N2][H2]3[NH3]2 Round your answer to 2 significant figures. Ked=
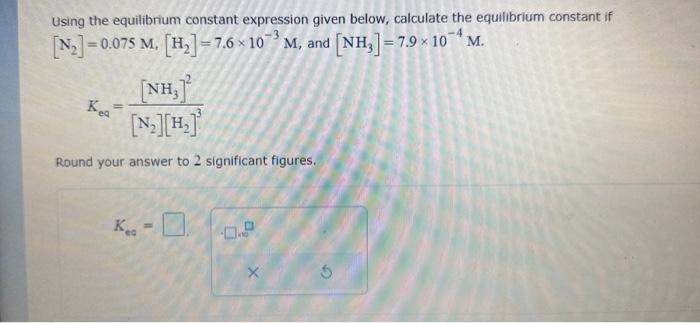
Using the equilibrium constant expression given below, calculate the equilibrium constant if [N2]=0.075M,[H2]=7.6103M, and [NH3]=7.9104M. Keq=[N2][H2]3[NH3]2 Round your answer to 2 significant figures. Ked=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


