Question: Using the following data at 879 K: A(s) + B(g) 2 C(g), Kci = 9 = (1) x103 5 E(g), Kc2 = 4 = (2)
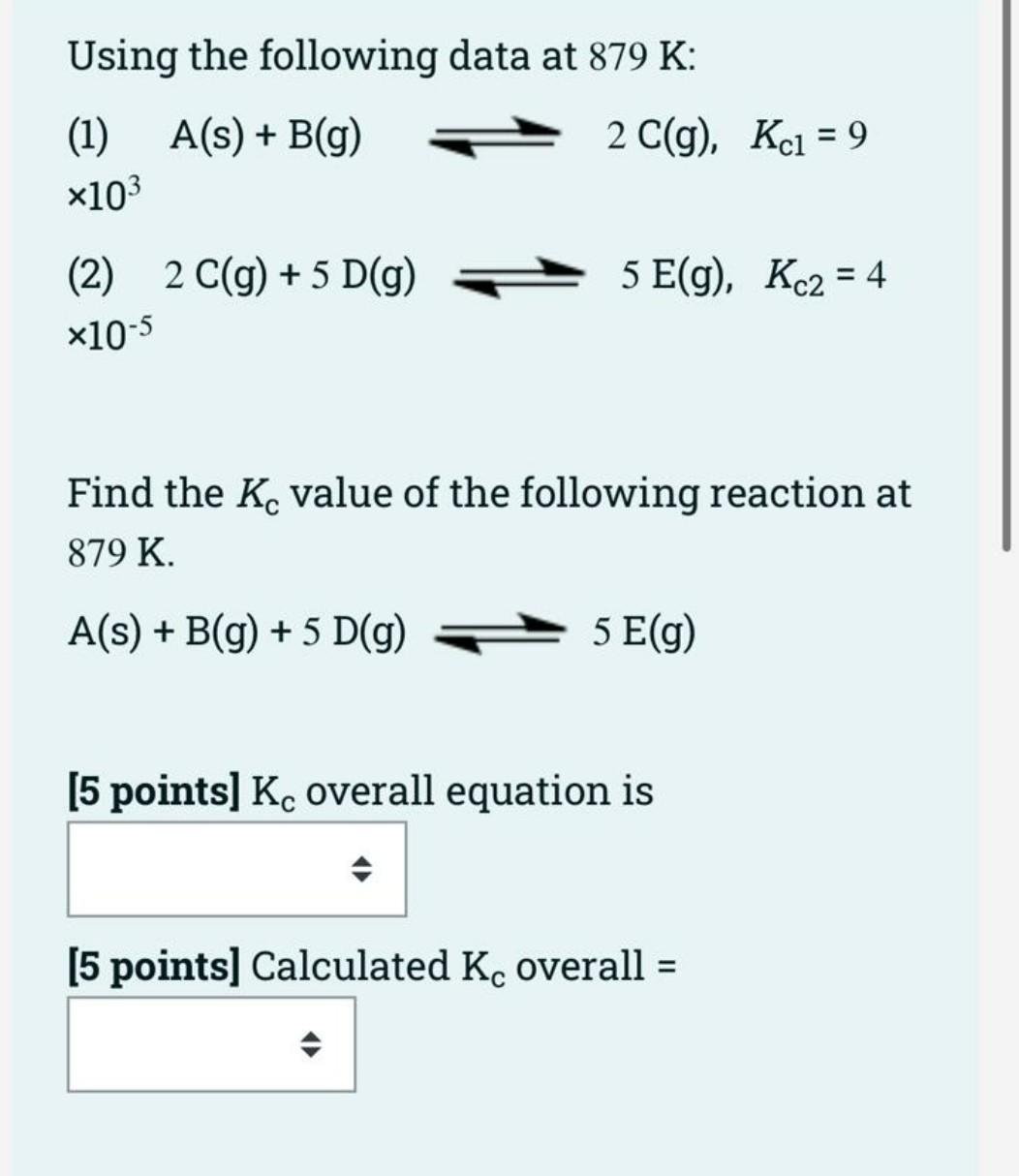
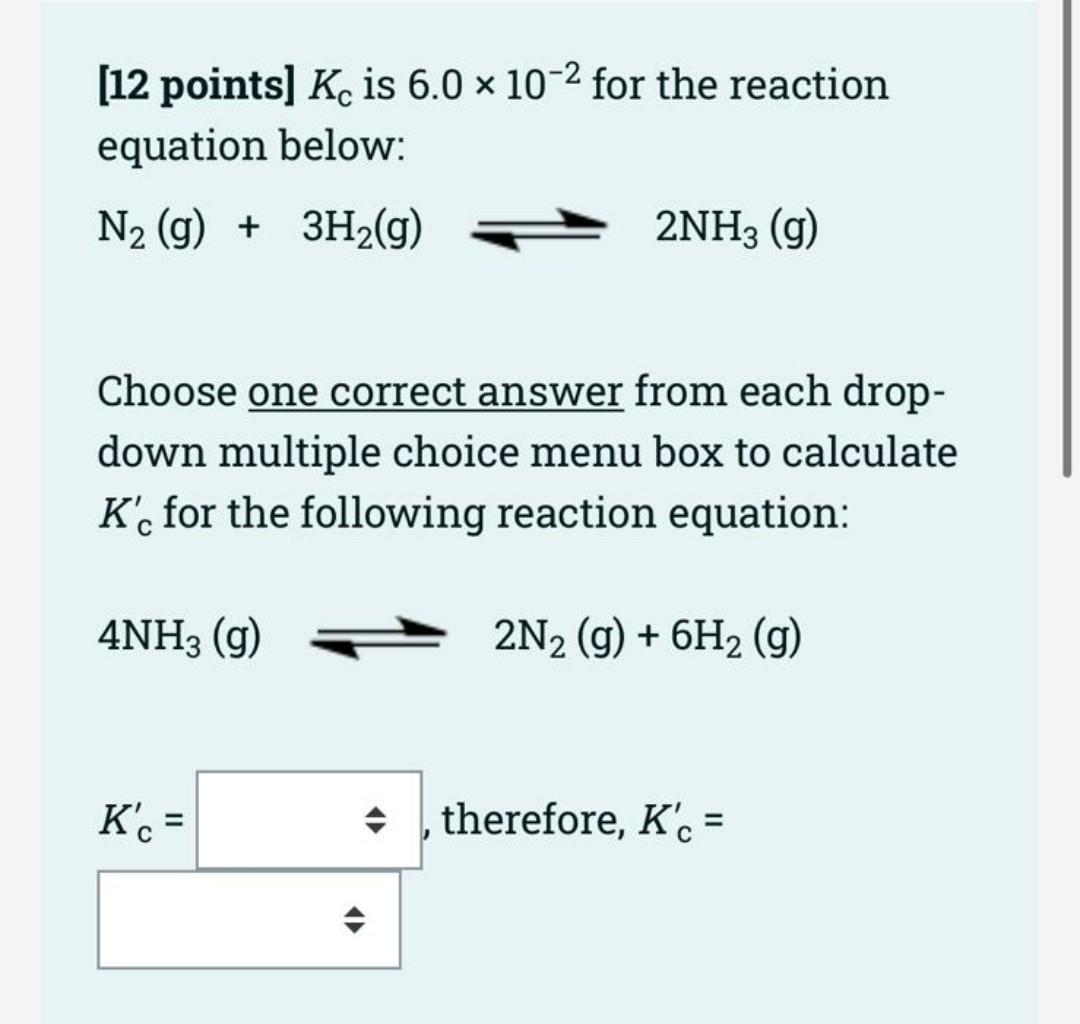
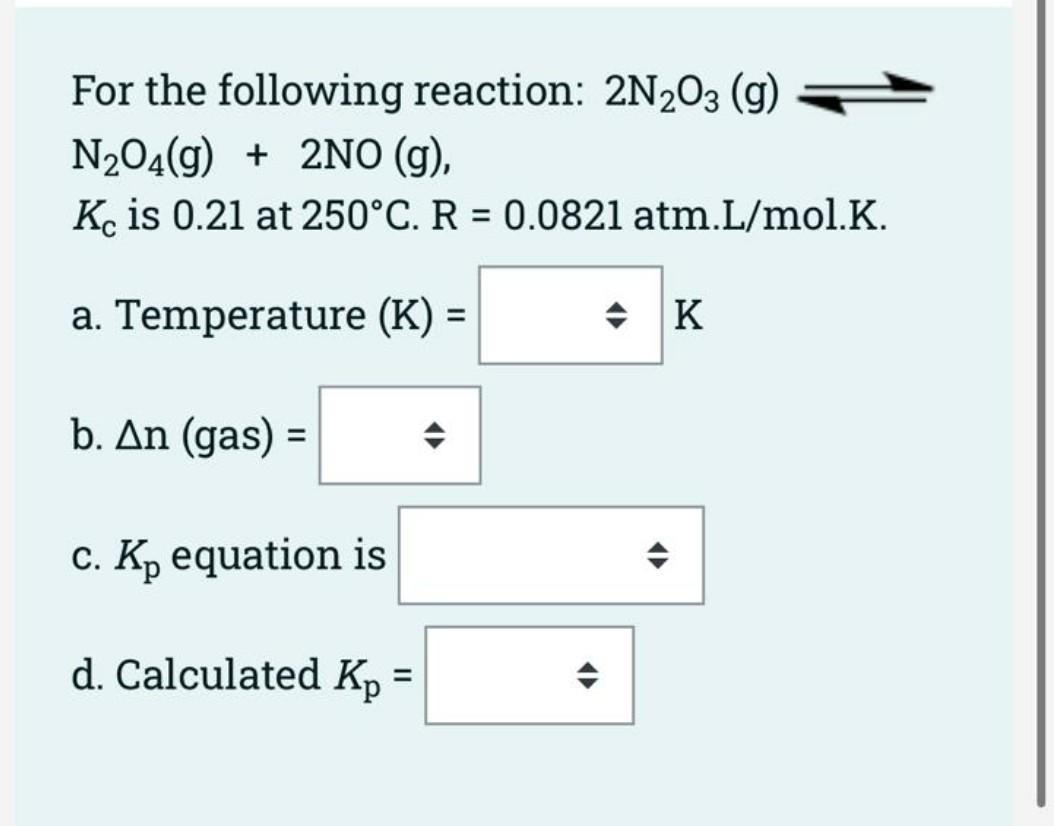
Using the following data at 879 K: A(s) + B(g) 2 C(g), Kci = 9 = (1) x103 5 E(g), Kc2 = 4 = (2) 2 C(g) + 5 D(g) x10-5 Find the Kc value of the following reaction at 879 K. A(s) + B(g) + 5 D(g) 5 E(g) [5 points) Kc overall equation is [5 points] Calculated Kc overall = (12 points] Kc is 6.0 x 10-2 for the reaction equation below: N2 (g) + 3H2(g) 2NH3 (g) Choose one correct answer from each drop- down multiple choice menu box to calculate K', for the following reaction equation: 4NH3 (9) 2N2 (g) + 6H2 (g) K' = , therefore, K's = For the following reaction: 2N203 (9) N204(g) + 2NO (g), Kc is 0.21 at 250C. R = 0.0821 atm.L/mol.K. = a. Temperature (K) = = * K b. An (gas) = c. Kp equation is d. Calculated Kp = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


