Question: = Using the formula for the hydrogen atom energy levels, En constant can be written in terms of fundamental quantities, ue4 1 the Rydberg 802h2
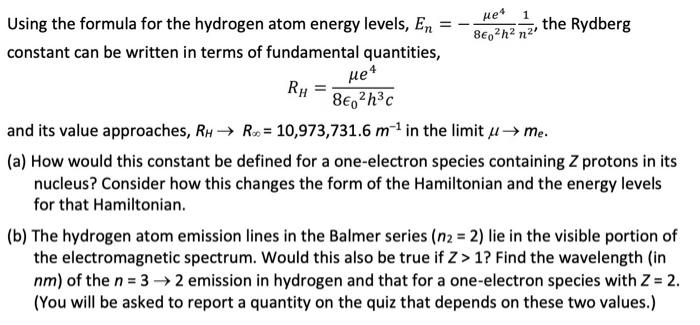
= Using the formula for the hydrogen atom energy levels, En constant can be written in terms of fundamental quantities, ue4 1 the Rydberg 802h2 n2 + RH 8, 2h3c and its value approaches, RH Rx = 10,973,731.6 m in the limit u me. (a) How would this constant be defined for a one-electron species containing Z protons in its nucleus? Consider how this changes the form of the Hamiltonian and the energy levels for that Hamiltonian. (b) The hydrogen atom emission lines in the Balmer series (n2 = 2) lie in the visible portion of the electromagnetic spectrum. Would this also be true if Z> 1? Find the wavelength (in nm) of the n = 3 2 emission in hydrogen and that for a one-electron species with Z = 2. (You will be asked to report a quantity on the quiz that depends on these two values.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


