Question: Using the given data we should answer the last page ... W_._-_ Datue of ohe I Inkmown Silste (14 nts) (graphs 4pts) Part (A): Freezing
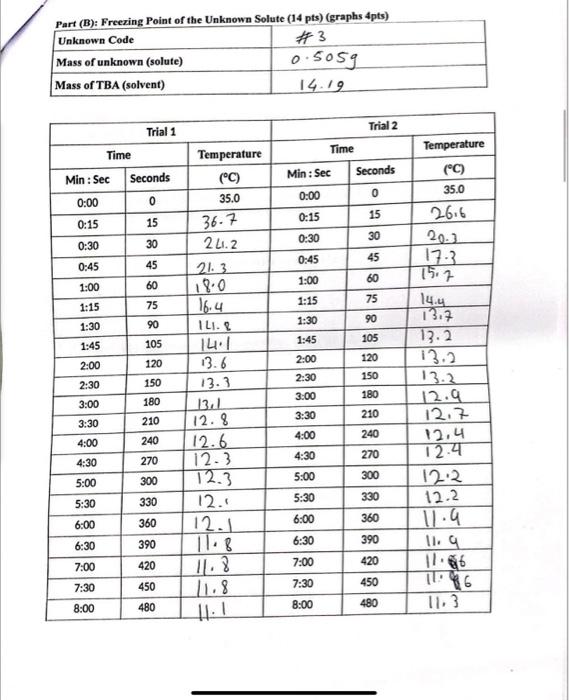
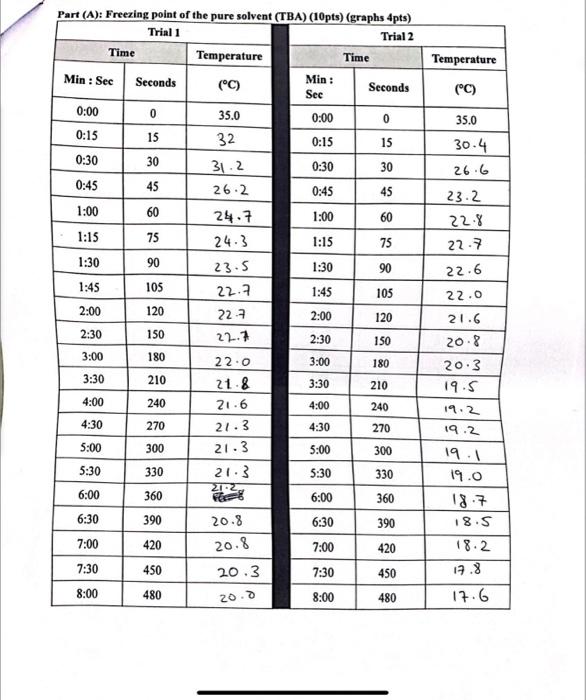

... W_._-_ Datue of ohe I Inkmown Silste (14 nts) (graphs 4pts) Part (A): Freezing point of the pure solvent (TBA) (10pts) (graphs 4pts) 1. Calculate the depression of the freezing point [in C] resulting from the addition of the unknown solute to the TBA. (4pts) 2. Use the given value of the freezing point depression constant Kr for TBA to calculate the molality of the TBA solution containing the unknown solute. (4pts) T=ik1mm=iKT= 3. Calculate the moles of unknown solute in the solution. (4pts) 4. Calculate the molar mass of the unknown solute. (4pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


