Question: using the given information and assuming that the mixture is ideal and binary, solve the prompt = log10 psat from the Antoine equation, B A-
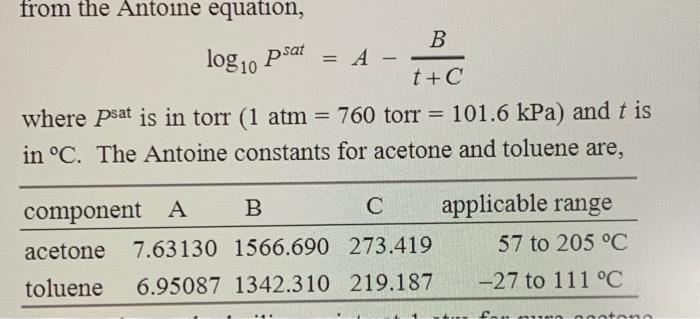

= log10 psat from the Antoine equation, B A- t+C where psat is in torr (1 atm = 760 torr = 101.6 kPa) and t is in C. The Antoine constants for acetone and toluene are, component A B C applicable range acetone 7.63130 1566.690 273.419 57 to 205 C toluene 6.95087 1342.310 219.187 -27 to 111 C 10 Calculate the liquid and vapor compositions for a 40. mol % acetone and 60. mol % toluene mixture at equilibrium with moles liquid / moles vapor = 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


