Question: Using the initial rates method and the experimental data, determine the rate law and the value of the rate constant for this reaction: 2NO(g)+Cl2(g)2NOCl(g)
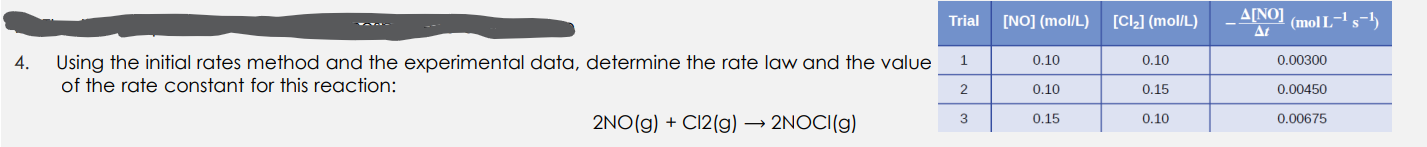
Using the initial rates method and the experimental data, determine the rate law and the value of the rate constant for this reaction: 2NO(g)+Cl2(g)2NOCl(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


