Question: 60 With reference to the reaction shown below and given the data in the table below: S208?- (aq) + 31 (aq) + 2SO42- (aq) +
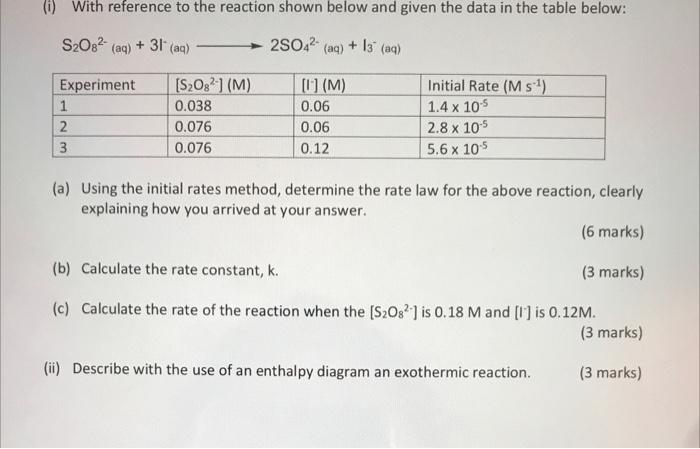
60 With reference to the reaction shown below and given the data in the table below: S208?- (aq) + 31 (aq) + 2SO42- (aq) + 13" (aq) Experiment 1 2 3 [S2082-1 (M) 0.038 0.076 0.076 [] (M) 0.06 0.06 0.12 Initial Rate (M :1) 1.4 x 105 2.8 x 10-5 5.6 x 105 (a) Using the initial rates method, determine the rate law for the above reaction, clearly explaining how you arrived at your answer. (6 marks) (b) Calculate the rate constant, k. (3 marks) (c) Calculate the rate of the reaction when the [S208?) is 0.18 M and [] is 0.12M. (3 marks) (ii) Describe with the use of an enthalpy diagram an exothermic reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


