Question: Using the line-bond structure for nicotine shown below: a. Write the molecular formula for nicotine. b. Indicate the total number of lone pairs missing from
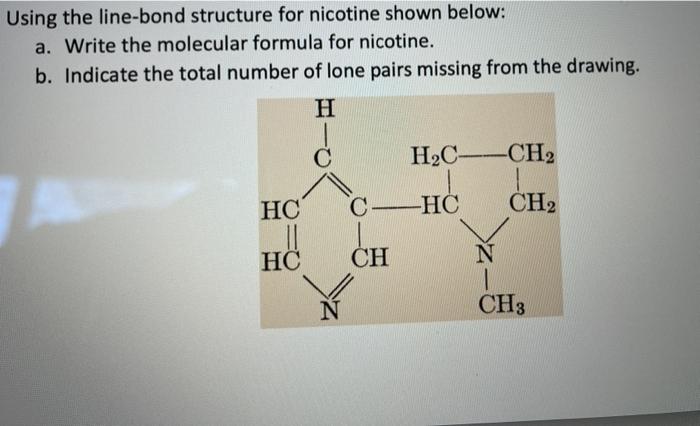
Using the line-bond structure for nicotine shown below: a. Write the molecular formula for nicotine. b. Indicate the total number of lone pairs missing from the drawing. H H2C --CH2 HC C-HC CH2 HC CH N 1 CH3 N
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


