Question: Using the Periodic Table (see below) to complete the information in the following table for the first 10 chemical elements: Element Symbol Atomic Atomic Weight
Using the Periodic Table (see below) to complete the information in the following table for the first 10 chemical elements:
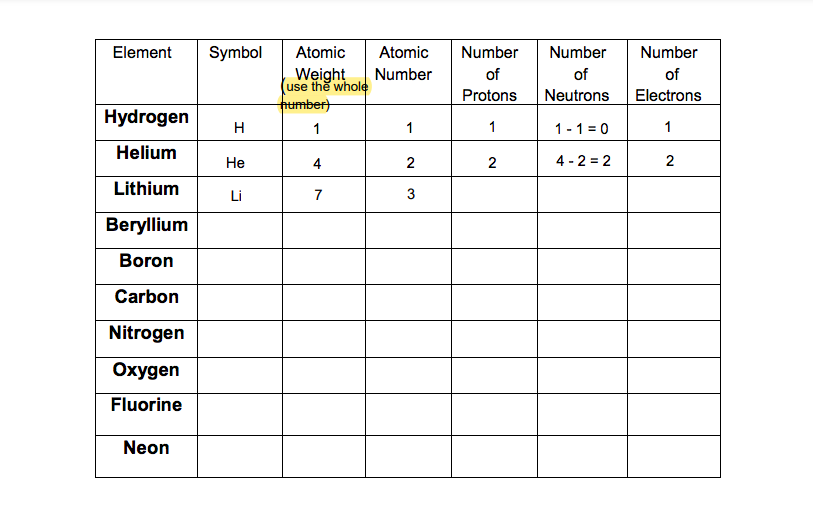

Element Symbol Atomic Atomic Weight Number use the whole Humber) 1 1 Number of Protons Number of Neutrons Number of Electrons Hydrogen H 1 1 - 1 = 0 1 Helium He 4 2 2 4-2 = 2 2 Lithium 7 3 Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Exercise #2: Electron Configurations Procedure: For the following elements, draw the electron configuration. Keep in mind that only two electrons can be placed in the first electron shell. Up to eight electrons can be placed in the second and third shell. Before placing electrons in the second shell, the first must be "full. Before placing electrons in the third shell, both the first and second must be tull (Figure 3.2). When drawing electron configuration for a given atom, there will be four orbitals available at each electron shell for those chemical atoms that we covered in Chapter 2 of the textbook, Each orbital can take a maximum of two electrons. At each electron shell, at least one electron needs to occupy each of the four orbitals first before pairing up the orbitals 1. Nitrogen KEY Proton Neutron Electron 2. Lithium (a) Hydrogen 1 proton ( O neutron (0) 1 electron (0) (b) Helium (H) 2 protoos (D) 2 neutrons (1) 2 electrons (0) 3. Phosphorus 4. Boron (c) Carbon (C) 6 protons (p) 6 neutrons (1) 6 electrons() Figure 3.2 Atomic structure 5. Oxygen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


