Question: Using the permeability coefficients for low density polyethylene and polypropylene in the table below, choose the correct statements. a. A pressure difference of 1000kPa would
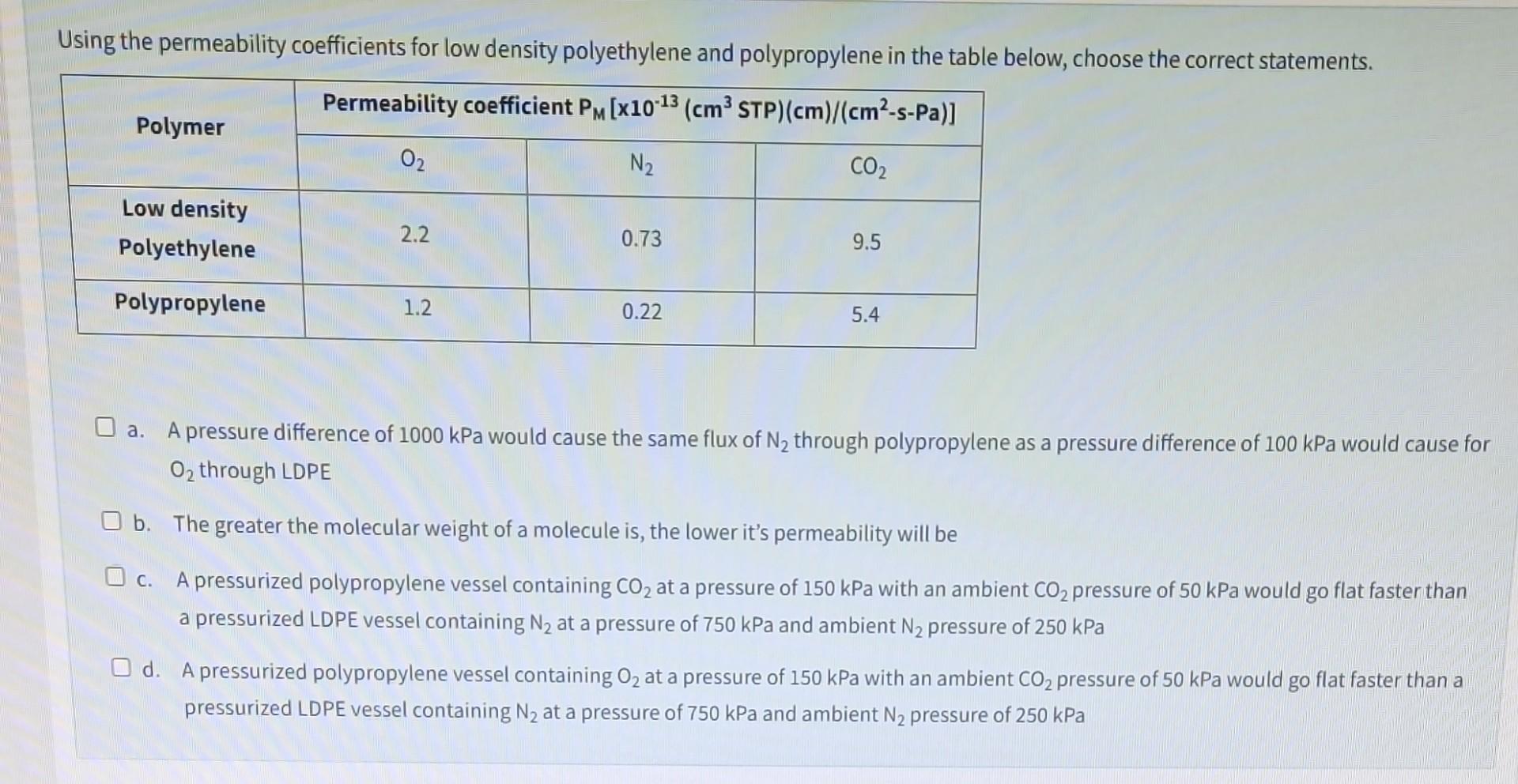
Using the permeability coefficients for low density polyethylene and polypropylene in the table below, choose the correct statements. a. A pressure difference of 1000kPa would cause the same flux of N2 through polypropylene as a pressure difference of 100kPa would cause for O2 through LDPE b. The greater the molecular weight of a molecule is, the lower it's permeability will be c. A pressurized polypropylene vessel containing CO2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa d. A pressurized polypropylene vessel containing O2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa Using the permeability coefficients for low density polyethylene and polypropylene in the table below, choose the correct statements. a. A pressure difference of 1000kPa would cause the same flux of N2 through polypropylene as a pressure difference of 100kPa would cause for O2 through LDPE b. The greater the molecular weight of a molecule is, the lower it's permeability will be c. A pressurized polypropylene vessel containing CO2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa d. A pressurized polypropylene vessel containing O2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


