Question: Using the provided table and the equation below, determine the heat of formation (in kJ/mol ) for PbS. 2PbS(s)+3O2(g)2SO2(g)+2PbO(s)H=828.4kJ/mol
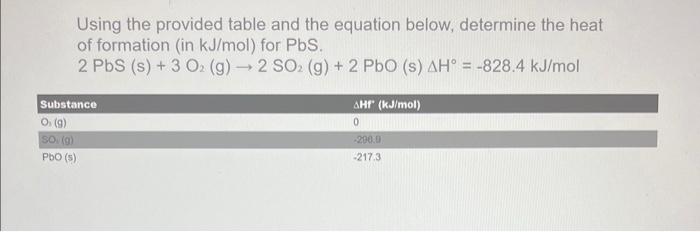
Using the provided table and the equation below, determine the heat of formation (in kJ/mol ) for PbS. 2PbS(s)+3O2(g)2SO2(g)+2PbO(s)H=828.4kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


