Question: Using the same reaction as before: ABC. (a) Write a system of differential equations to models the reaction (i.e. write an ODE to model,
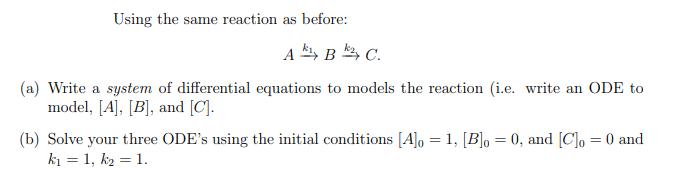
Using the same reaction as before: ABC. (a) Write a system of differential equations to models the reaction (i.e. write an ODE to model, [A], [B], and [C]. (b) Solve your three ODE's using the initial conditions [A]o = 1, [B]o = 0, and [C] = 0 and k = 1, k = 1.
Step by Step Solution
3.41 Rating (160 Votes )
There are 3 Steps involved in it
a The given reaction is A B C Lets denote the concentrations of A B and C as A B and C respectively ... View full answer

Get step-by-step solutions from verified subject matter experts


