Question: Using the table below, determine the boiling point of a solution made by dissolving 51.8 g of bromobenzene, C6H5Br, in 107.8 mL of chloroform CHCl3.
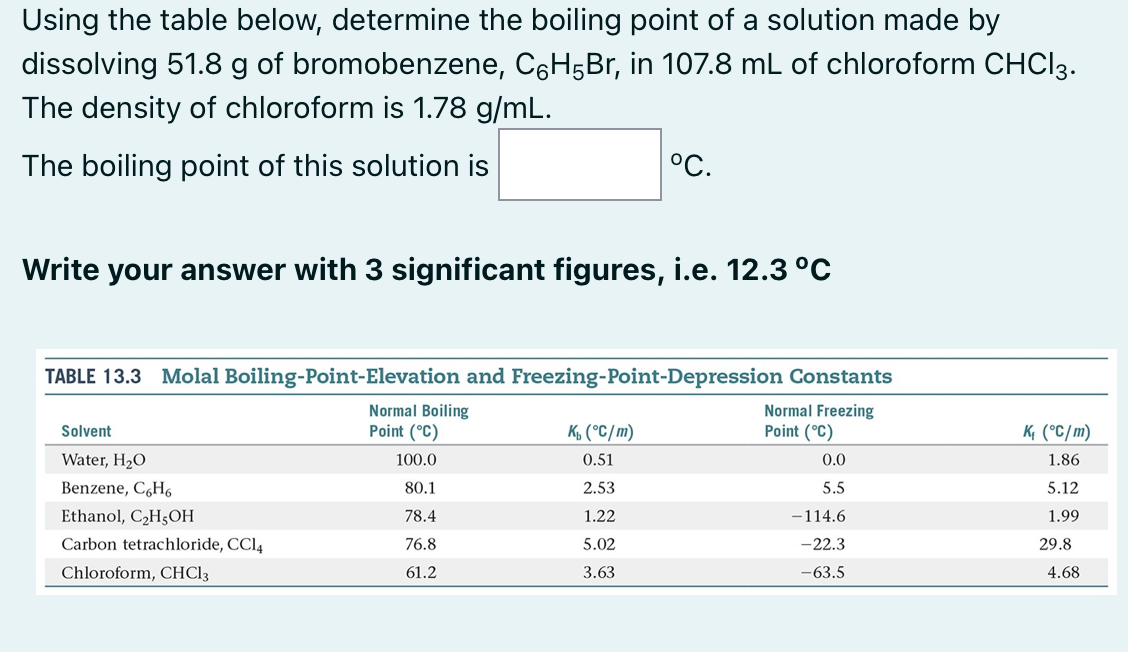 Using the table below, determine the boiling point of a solution made by dissolving 51.8 g of bromobenzene, C6H5Br, in 107.8 mL of chloroform CHCl3. The density of chloroform is 1.78 g/mL.
Using the table below, determine the boiling point of a solution made by dissolving 51.8 g of bromobenzene, C6H5Br, in 107.8 mL of chloroform CHCl3. The density of chloroform is 1.78 g/mL.
Using the table below, determine the boiling point of a solution made by dissolving 51.8g of bromobenzene, C6H5Br, in 107.8mL of chloroform CHCl3. The density of chloroform is 1.78g/mL. The boiling point of this solution is C. Write your answer with 3 significant figures, i.e. 12.3C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


