Question: using the titration values i got, please answer the questions 2-4 please 1st 2nd Titration final und Inital read Vol added 18. 17cm3 0.00 cm3
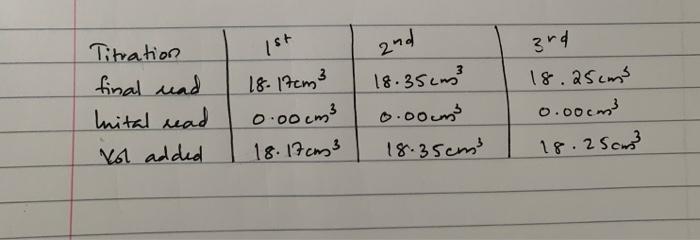

1st 2nd Titration final und Inital read Vol added 18. 17cm3 0.00 cm3 18. 17cm3 18.35cm? 0.00 cm 18.35cm 3rd 18.25cm 0.com 18.250 Part 2 Determination of the concentration of a H2O2 solution using thiosulphate solution (you need accurate value of concentration)(approx. 0.1M). Pipette 10 cm' of the original stock H,02("10 vol") solution into a 250cm volumetric flask and make up with distilled water. Label this as solution B. Pipette 25 cm of this solution into a solution containing 1g of KI in 25 cm of 4 M H2SO4 and 25 cm of water with constant stirring. Allow to stand for 15 min. Titrate the liberated iodine with Na2S2O3 (0.1 M) as follows (x 3 times): . Titrate until the blue-black colours turns pale yellow. Add approx. 3-4 drops of starch solution, [3 or so drops should suffice) and continue titrating until the dark-blue colour has almost faded. Add 10cm of 15 % potassium thiocyanate solution. The dark blue colour returns. Titrate to a colourless end-point, (take care not to over titrate). Repeat titration to obtain agreeing titre values to within 0.1 cm In your lab-book (1) Write out equations for the reactions involved (2) Calculate the molarity of the hydrogen peroxide in solution B. (3) Use the answer to (2) to calculate the volume strength of the hydrogen peroxide in the original solution (4) Calculate the concentration of Ho, in g dm in the original solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


