Question: Using the Values from the ICE table, construct the expression for the solubility constant Determine the value of Ksp for AgIO3 by constructing an ICE
 Using the Values from the ICE table, construct the expression for the solubility constant
Using the Values from the ICE table, construct the expression for the solubility constant
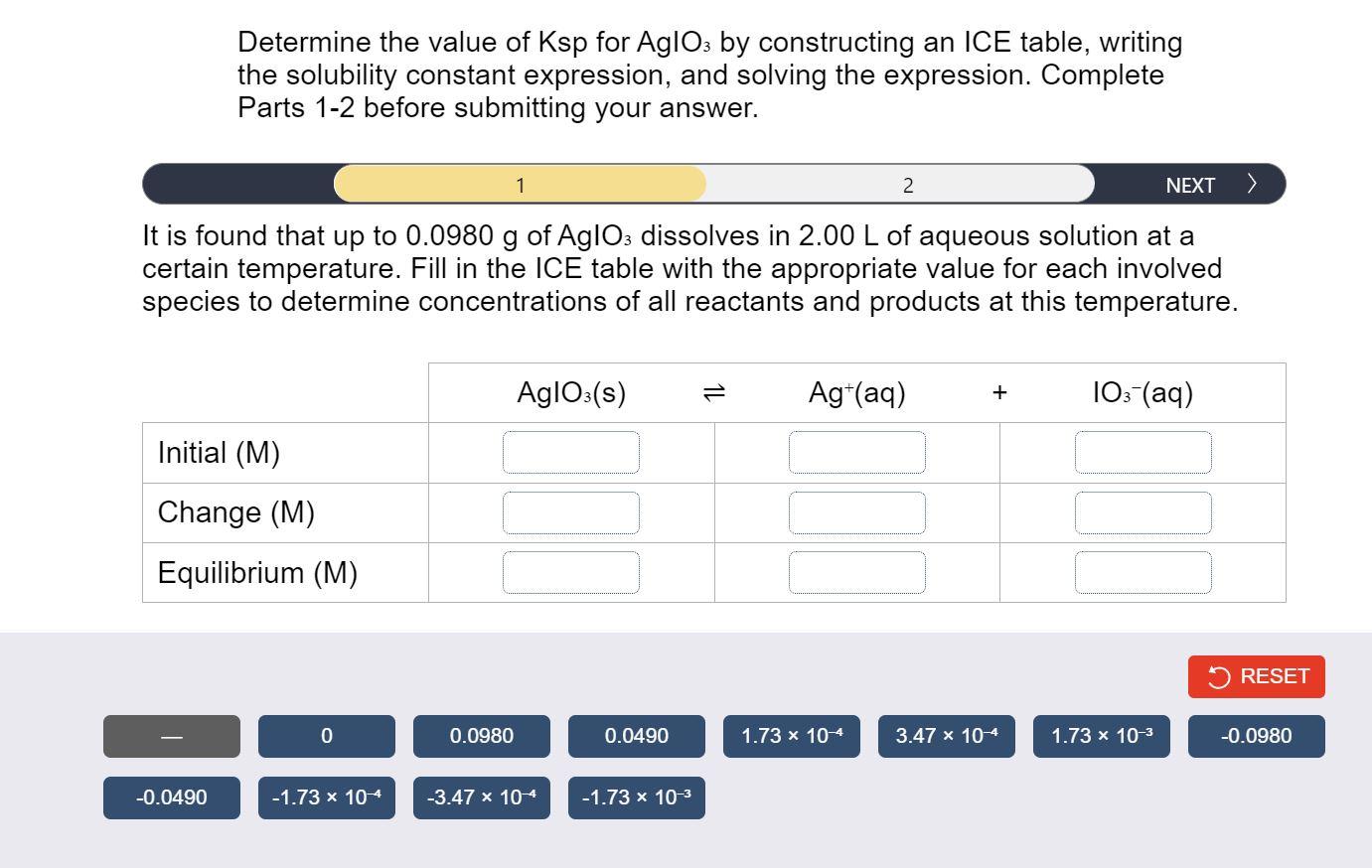
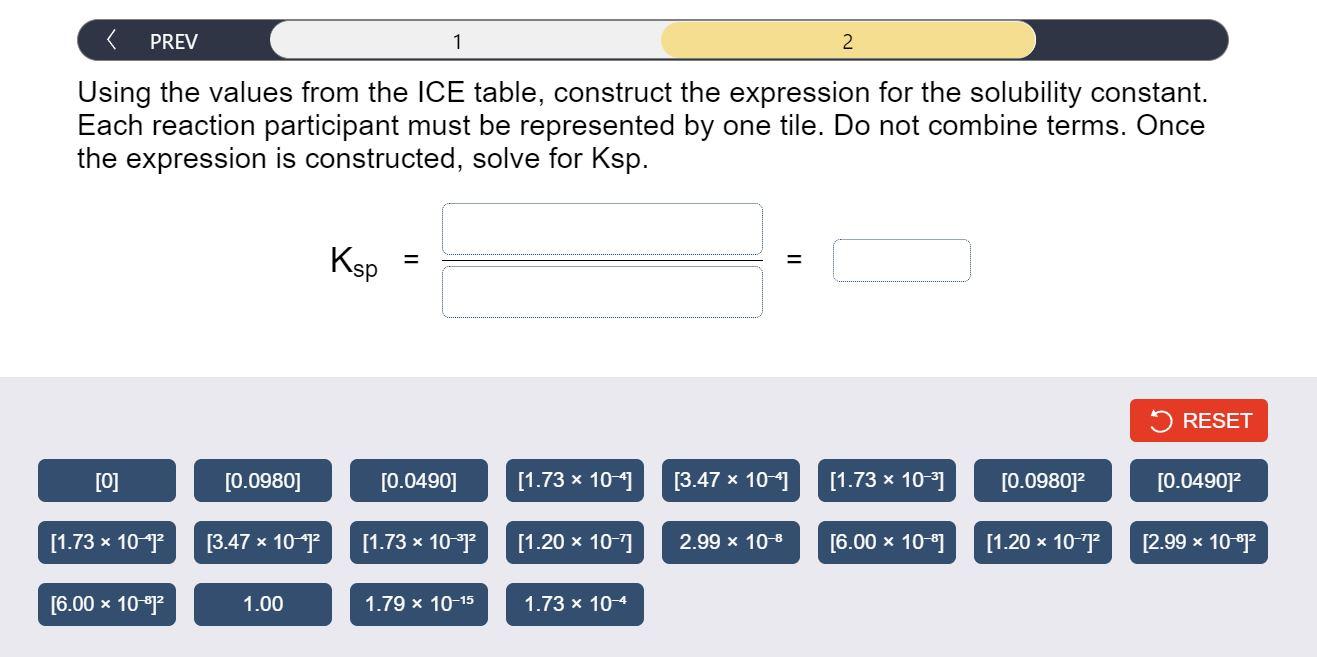
Determine the value of Ksp for AgIO3 by constructing an ICE table, writing the solubility constant expression, and solving the expression. Complete Parts 1-2 before submitting your answer. It is found that up to 0.0980g of AgIO3 dissolves in 2.00L of aqueous solution at a certain temperature. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products at this temperature. Using the values from the ICE table, construct the expression for the solubility constant. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Ksp. Ksp=(1)=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


