Question: Using VSEPR theory, determine the electron- group geometry and molecular shape of the following substances: (a) BF3 (b) NF3 (c) CF4 (d) CF,H2
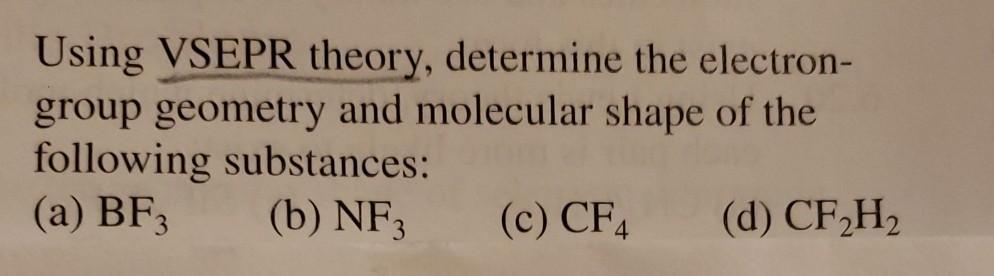
Using VSEPR theory, determine the electron- group geometry and molecular shape of the following substances: (a) BF3 (b) NF3 (c) CF4 (d) CF,H2
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


