Question: v For each example, specify whether the two structures are resonance contributors to the same resonance hybrid. a) b) c) H3COC+H2 No more group attempts
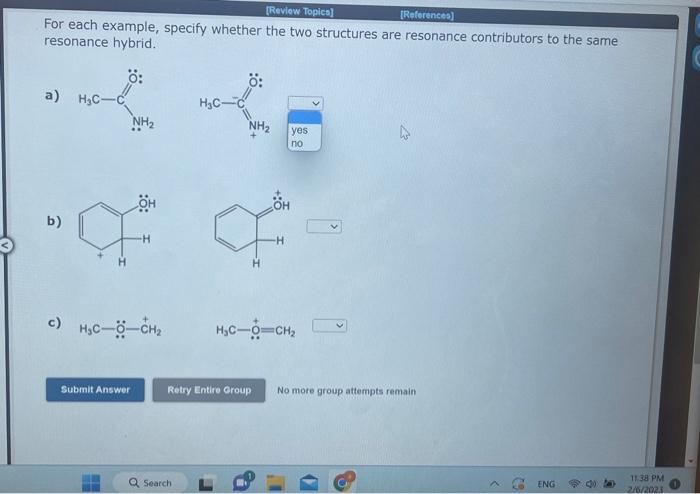
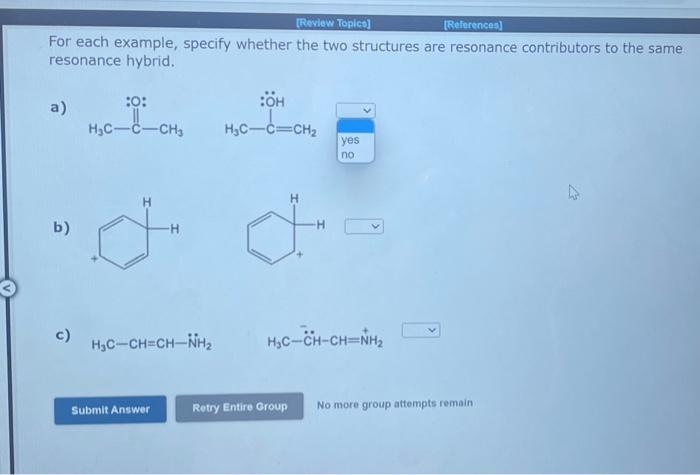
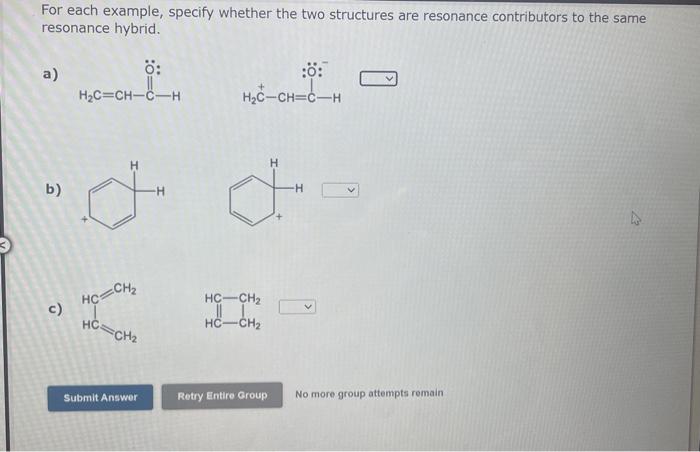
For each example, specify whether the two structures are resonance contributors to the same resonance hybrid. a) b) c) H3COC+H2 No more group attempts remain For each example, specify whether the two structures are resonance contributors to the same resonance hybrid. a) b) c) H3CCH=CHNH2 No more group attempts remain For each example, specify whether the two structures are resonance contributors to the same resonance hybrid. a) b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


