Question: For each example, specify whether the two structures are resonance contributors to the same resonance hybrid. a) b) c) For each example, specify whether the
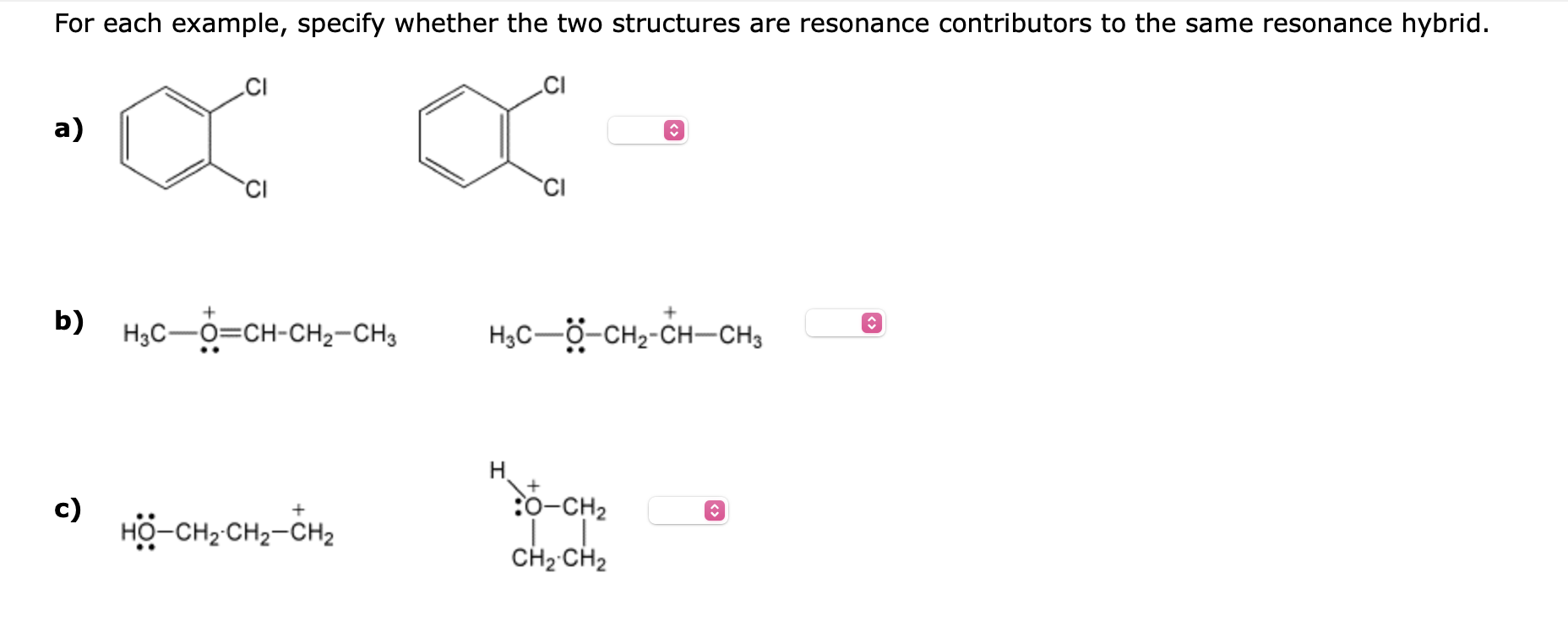
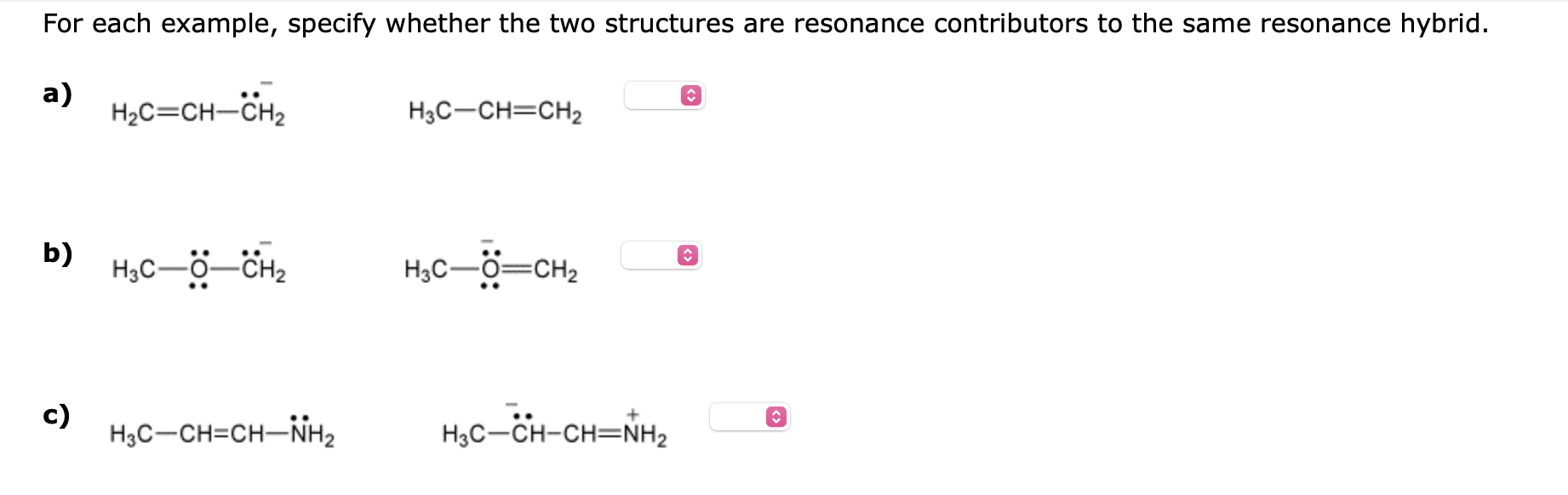
For each example, specify whether the two structures are resonance contributors to the same resonance hybrid. a) b) c) For each example, specify whether the two structures are resonance contributors to the same resonance hybrid. a) H2C=CHCH2 H3CCH=CH2 b) c) H3CCH=CHNH2 H3CCHCH=N+H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


