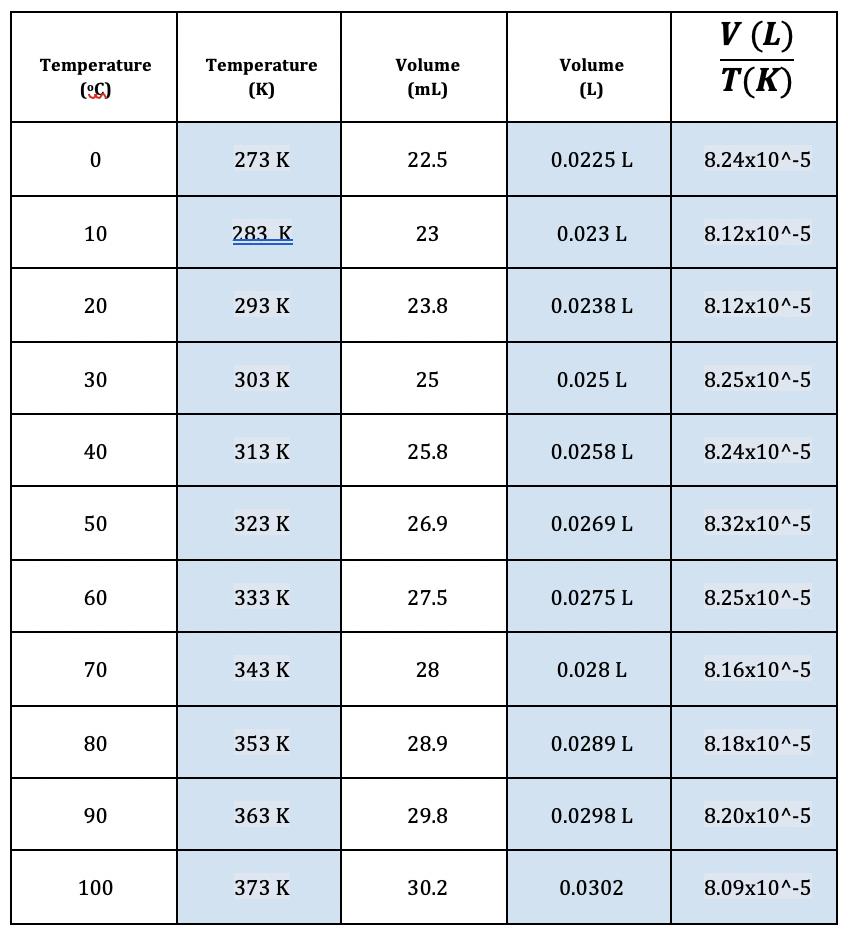
Question: V (L) T(K) Volume Volume Temperature (C) Temperature (K) (mL) (L) 273 K 22.5 0.0225 L 8.24x10^-5 10 283 K 23 0.023 L 8.12x10^-5
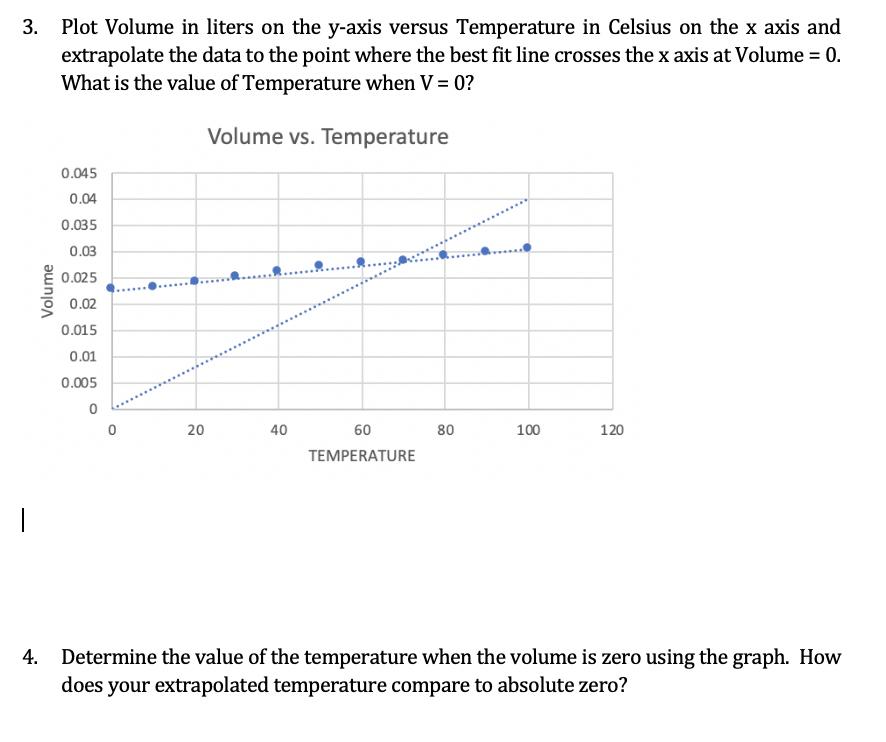
V (L) T(K) Volume Volume Temperature (C) Temperature (K) (mL) (L) 273 K 22.5 0.0225 L 8.24x10^-5 10 283 K 23 0.023 L 8.12x10^-5 20 293 K 23.8 0.0238 L 8.12x10^-5 30 303 K 25 0.025 L 8.25x10^-5 40 313 K 25.8 0.0258 L 8.24x10^-5 50 323 K 26.9 0.0269 L 8.32x10^-5 60 333 K 27.5 0.0275 L 8.25x10^-5 70 343 K 28 0.028 L 8.16x10^-5 80 353 K 28.9 0.0289 L 8.18x10^-5 90 363 K 29.8 0.0298 L 8.20x10^-5 100 373 K 30.2 0.0302 8.09x10^-5 Plot Volume in liters on the y-axis versus Temperature in Celsius on the x axis and extrapolate the data to the point where the best fit line crosses the x axis at Volume = 0. What is the value of Temperature when V= 0? %3D Volume vs. Temperature 0.045 0.04 0.035 0.03 0.025 0.02 0.015 0.01 0.005 20 40 60 80 100 120 TEMPERATURE 4. Determine the value of the temperature when the volume is zero using the graph. How does your extrapolated temperature compare to absolute zero? 3. Volume
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
4 Line intersect B... View full answer

Get step-by-step solutions from verified subject matter experts


