Question: van der Waals EOS: P=VbRTV2a a) Differentiate the van der Waals equation of state with respect to the molar volume. b) Using the fact that:

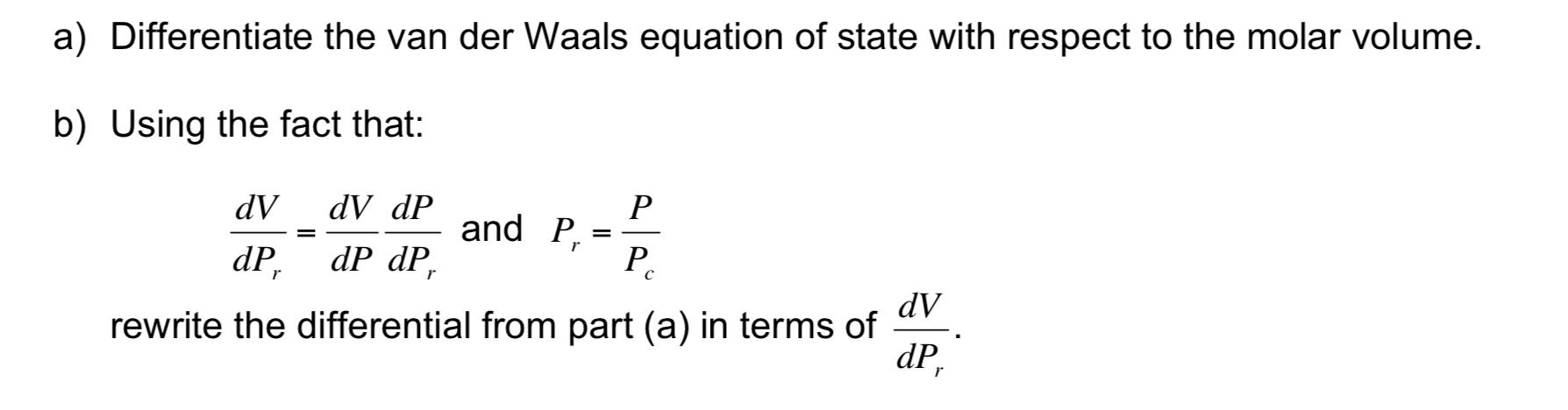

van der Waals EOS: P=VbRTV2a a) Differentiate the van der Waals equation of state with respect to the molar volume. b) Using the fact that: dPrdV=dPdVdPrdPandPr=PcP rewrite the differential from part (a) in terms of dPrdV. c) Use the resulting differential equation from part (b) to calculate the compressibility factor chart for CO2 at Tr=1.2 and 3.0 (note Tr=T/Tc ) over the reduced pressure range of 0.1Pr15. Generate a plot with your results (clearly labeled). Also, report the final values for the compressibility factors at Pr=15
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


