Question: Vanadium Pentaoxide can be used as a catalyst for the following reaction S O 2 + 1 2 O 2 l o n g r
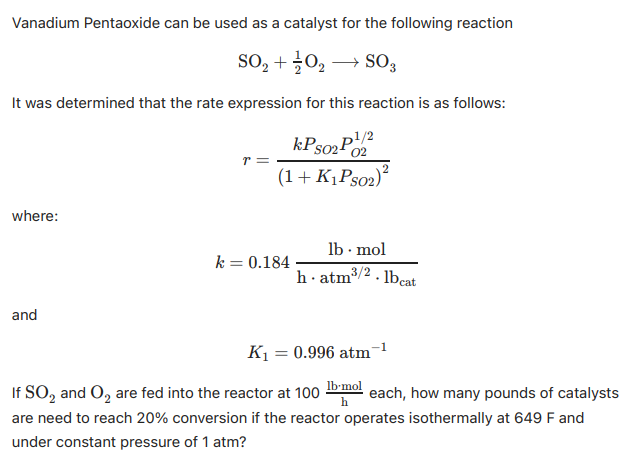
Vanadium Pentaoxide can be used as a catalyst for the following reaction
It was determined that the rate expression for this reaction is as follows:
where:
and
If and are fed into the reactor at each, how many pounds of catalysts
are need to reach conversion if the reactor operates isothermally at and
under constant pressure of atm?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


