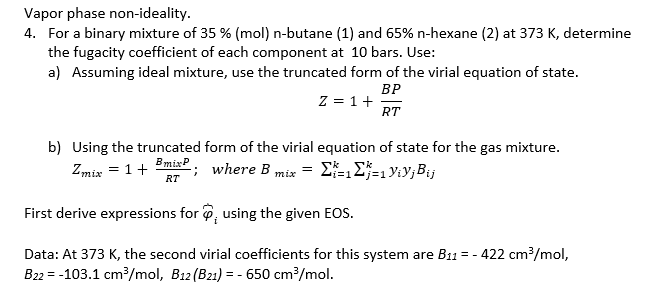
Question: Vapor phase non - ideality. For a binary mixture of 3 5 % ( mol ) n - butane ( 1 ) and 6 5
Vapor phase nonideality.
For a binary mixture of mol nbutane and nhexane at determine
the fugacity coefficient of each component at bars. Use:
a Assuming ideal mixture, use the truncated form of the virial equation of state.
b Using the truncated form of the virial equation of state for the gas mixture.
; where
First derive expressions for tilde using the given EOS.
Data: At the second virial coefficients for this system are
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


