Question: Verify that the composition in the phase is approximately 94 mol% a and 6 mol% b and the composition in the phase is the opposite.
Verify that the composition in the phase is approximately 94 mol% a and 6 mol% b and the composition in the phase is the opposite.
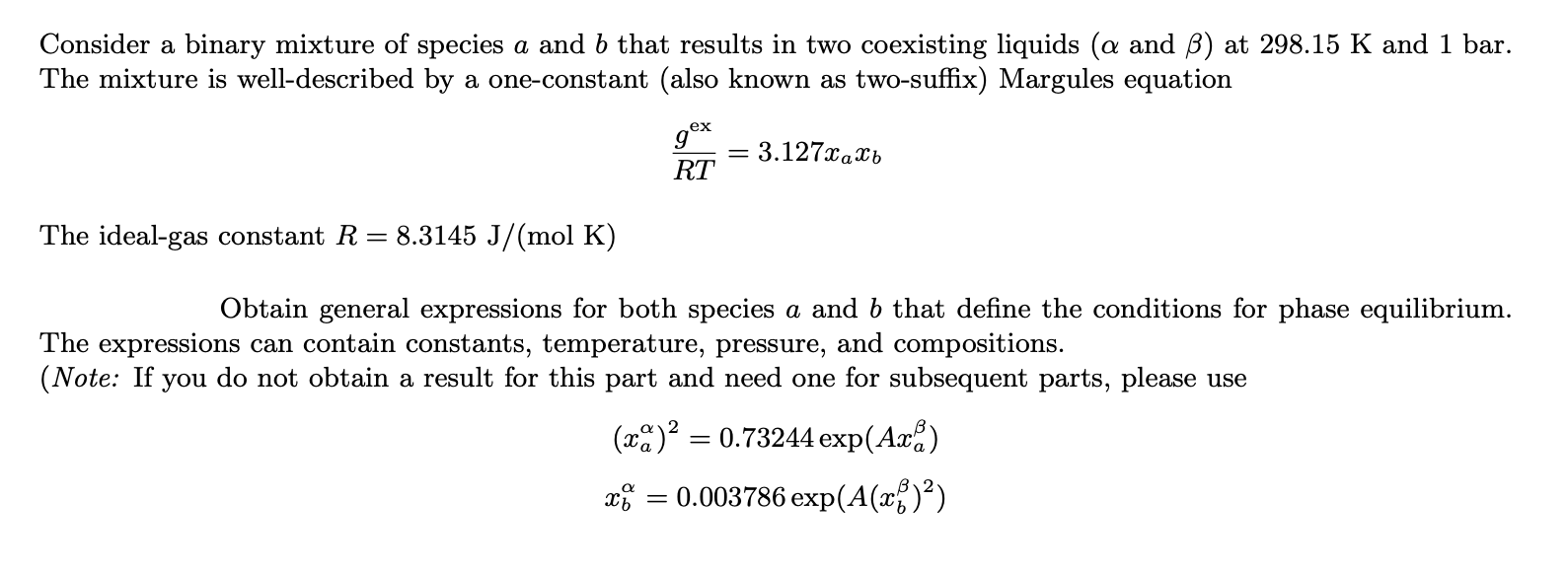
Consider a binary mixture of species a and b that results in two coexisting liquids ( and ) at 298.15K and 1 bar. The mixture is well-described by a one-constant (also known as two-suffix) Margules equation RTgex=3.127xaxb The ideal-gas constant R=8.3145J/(molK) Obtain general expressions for both species a and b that define the conditions for phase equilibrium. The expressions can contain constants, temperature, pressure, and compositions. (Note: If you do not obtain a result for this part and need one for subsequent parts, please use (xa)2=0.73244exp(Axa)xb=0.003786exp(A(xb)2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


