Question: Verify that your sample is completely dry Check your data. Reevaluate your calculations. Collected Volume sodium carbonate (ml) Molarity sodium carbonate (M) Volume calcium
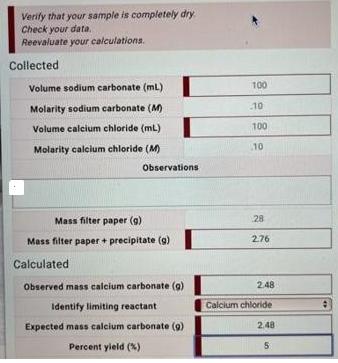
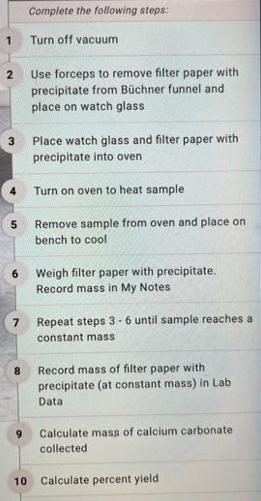
Verify that your sample is completely dry Check your data. Reevaluate your calculations. Collected Volume sodium carbonate (ml) Molarity sodium carbonate (M) Volume calcium chloride (mL) Molarity calcium chloride (M) Mass filter paper (g) Mass filter paper + precipitate (g) Calculated Observed mass calcium carbonate (g) Identify limiting reactant Expected mass calcium carbonate (9) Percent yield (%) Observations 100 10 100 10 28 276 2.48 Calcium chloride 2,48 5 1 2 3 5 6 7 8 10 Complete the following steps: Turn off vacuum Use forceps to remove filter paper with precipitate from Bchner funnel and place on watch glass Place watch glass and filter paper with precipitate into oven Turn on oven to heat sample. Remove sample from oven and place on bench to cool Weigh filter paper with precipitate. Record mass in My Notes Repeat steps 3-6 until sample reaches a constant mass Record mass of filter paper with precipitate (at constant mass) in Lab Data Calculate mass of calcium carbonate collected Calculate percent yield
Step by Step Solution
There are 3 Steps involved in it
Balanced Reaction NaCO3 Call CaCO3 2Nacl Molarity of N... View full answer

Get step-by-step solutions from verified subject matter experts


