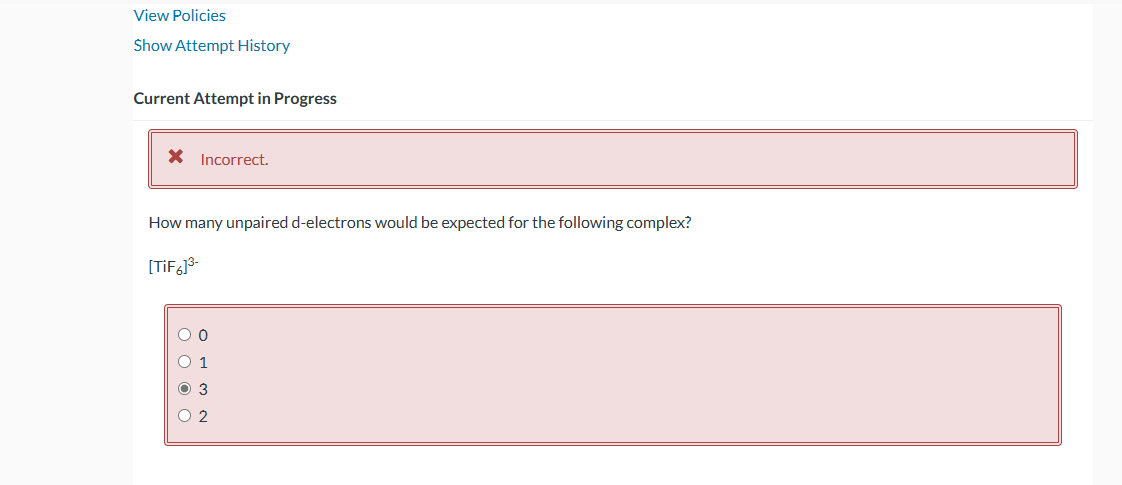
Question: View Policies Show Attempt History Current Attempt in Progress Incorrect. How many unpaired d-electrons would be expected for the following complex? [TiF6]3 0 1 3
![many unpaired d-electrons would be expected for the following complex? [TiF6]3 0](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f975d00c0fd_65566f975cfab10c.jpg)
![1 3 2 Sketch the d-orbital energy level diagrams for [Fe(OH2)6]3+ and](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f975d0976a9_65666f975d049bf5.jpg)
![[Fe(CN)6]3. Use your diagrams to answer the questions. Correct. The aqua complex](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f975d11cfc7_65666f975d0c3dac.jpg)
View Policies Show Attempt History Current Attempt in Progress Incorrect. How many unpaired d-electrons would be expected for the following complex? [TiF6]3 0 1 3 2 Sketch the d-orbital energy level diagrams for [Fe(OH2)6]3+ and [Fe(CN)6]3. Use your diagrams to answer the questions. Correct. The aqua complex is spin. The aqua complex has unpaired electron(s). Sketch the d-orbital energy level diagrams for [Fe(OH2)6]3+ and [Fe(CN)6]3. Use your diagrams to answer the questions. Correct. The aqua complex is spin. The aqua complex has unpaired electron(s). Correct. The cyano complex is spin. The cyano complex has unpaired electron(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


