Question: W diffusion reaction x=a(1-z/L) x Az W a diffusion Z=0 reaction. z+Az 8 is very small, so that cos 8=1 z-L Corrugated surfaces such
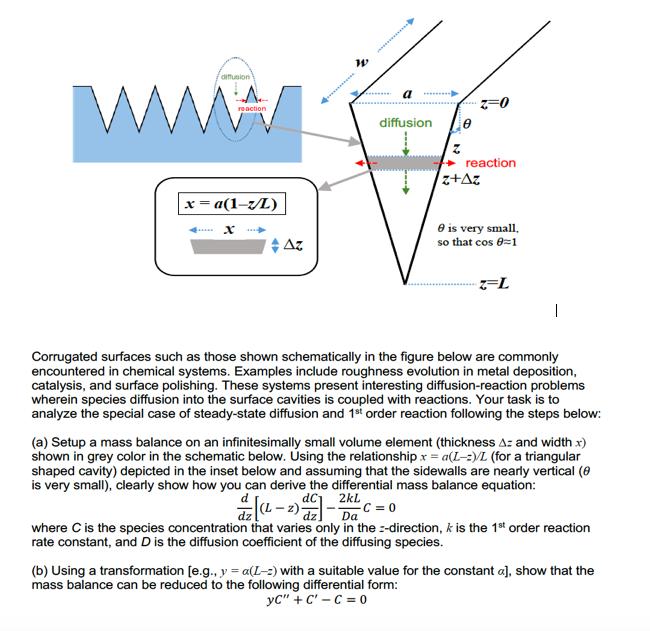
W diffusion reaction x=a(1-z/L) x Az W a diffusion Z=0 reaction. z+Az 8 is very small, so that cos 8=1 z-L Corrugated surfaces such as those shown schematically in the figure below are commonly encountered in chemical systems. Examples include roughness evolution in metal deposition, catalysis, and surface polishing. These systems present interesting diffusion-reaction problems wherein species diffusion into the surface cavities is coupled with reactions. Your task is to analyze the special case of steady-state diffusion and 1st order reaction following the steps below: (a) Setup a mass balance on an infinitesimally small volume element (thickness A: and width x) shown in grey color in the schematic below. Using the relationship x = a(L-:)/L (for a triangular shaped cavity) depicted in the inset below and assuming that the sidewalls are nearly vertical (8 is very small), clearly show how you can derive the differential mass balance equation: dC 2kL (1-2) - Da -C=0 where C is the species concentration that varies only in the direction, k is the 1st order reaction rate constant, and D is the diffusion coefficient of the diffusing species. (b) Using a transformation [e.g., y = a(L-2) with a suitable value for the constant a], show that the mass balance can be reduced to the following differential form: yC" + C-C=0
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
2 X1 X1 addeve To be designed using number of halbad... View full answer

Get step-by-step solutions from verified subject matter experts


