Question: Warning! The Surgeon General has determined that this problem is hazardous to your health. The oxidation numbers of Cu and Bi in high-temperature superconductors of

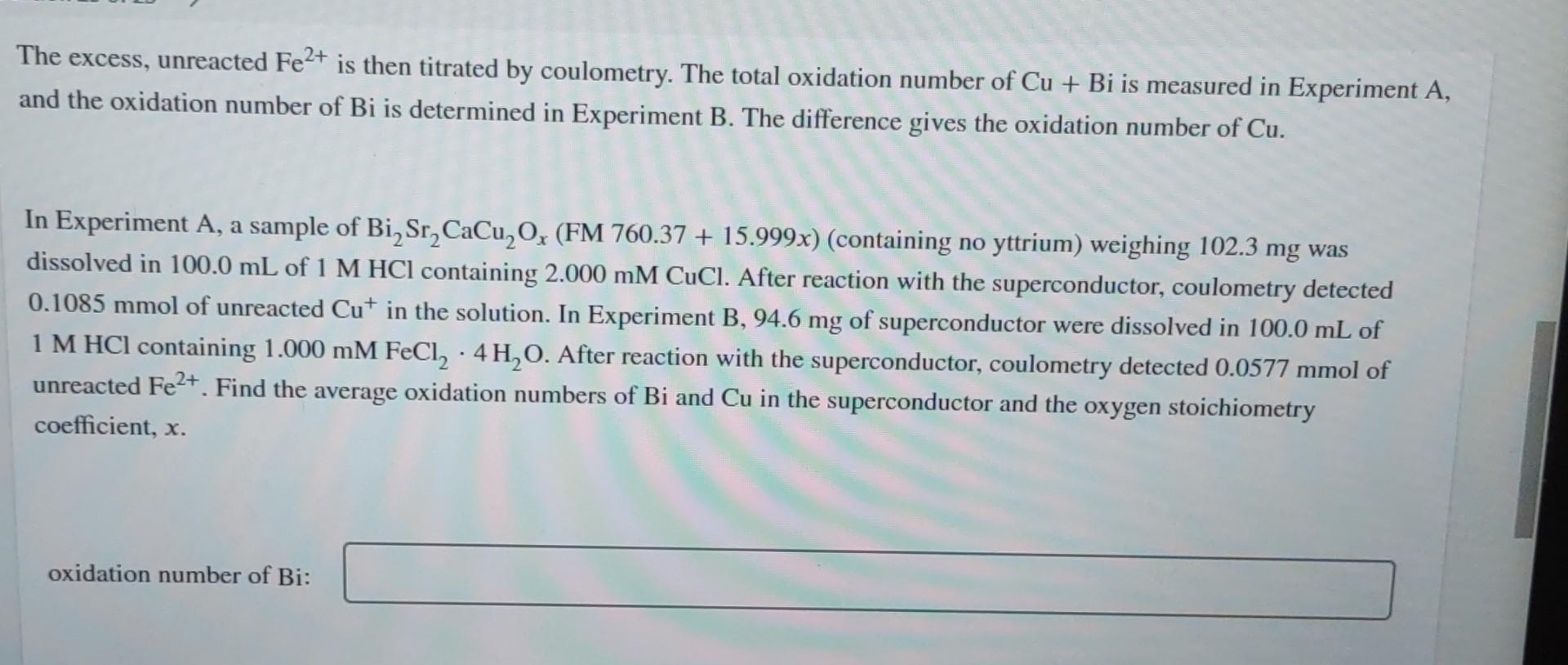
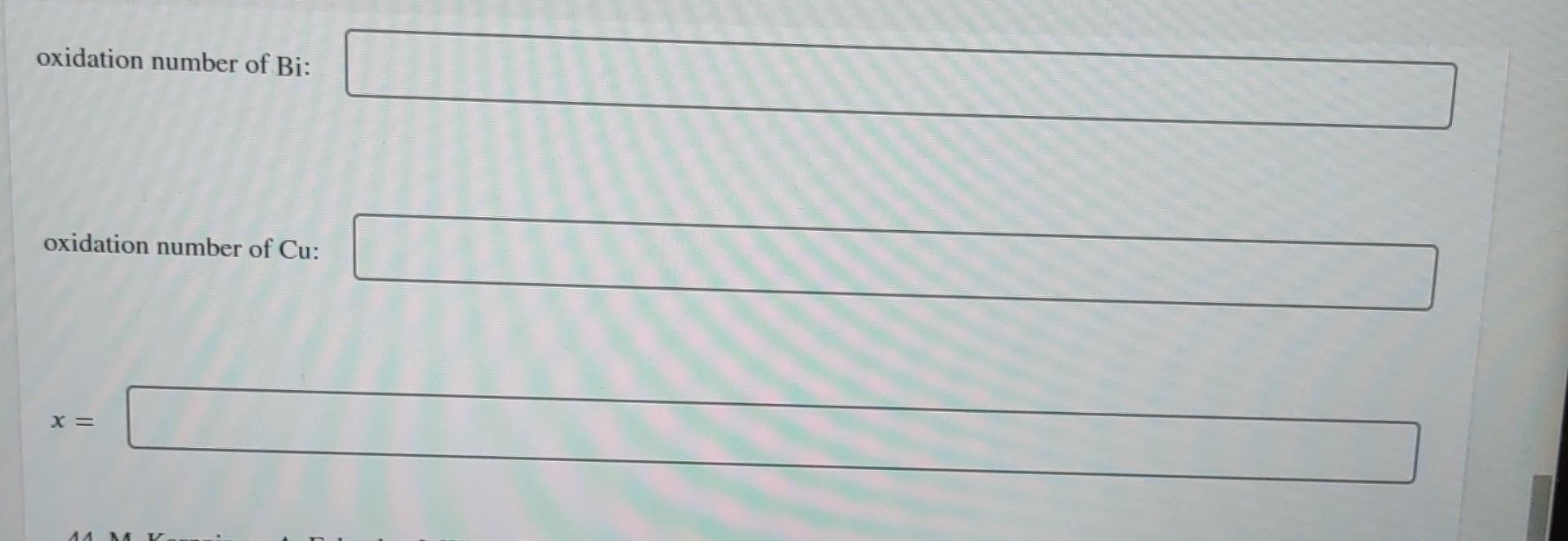
Warning! The Surgeon General has determined that this problem is hazardous to your health. The oxidation numbers of Cu and Bi in high-temperature superconductors of the type Bi,Sr (Cao.8 Yo.2)CuOx (which could contain Cu+, Cu+, Bi+, and Bi5 +) can be measured by the following procedure.44 In Experiment A, the superconductor is dissolved in 1 M HCl containing excess 2 mM CuCl. Bi5+ (written as BiO3) and Cu+ consume Cut to make Cu+: 3+ BiO3 + 2 Cut + 4H+ BiO+ + 2 Cu+ + 2HO (1) Cu+ + Cut 2 Cu+ (2) The excess, unreacted Cut is then titrated by coulometry. In Experiment B, the superconductor is dissolved in 1 M HCl containing excess 1 mM FeCl - 4HO. BiS+ reacts with the Fe+ but Cu+ does not react with Fe+.45 3+ BiO3 + 2Fe+ + 4H+ BiO+ + 2 Fe+ + 2HO (3) Cu+ + HO Cu+ + 0 0 + H+ 2+ (4) The excess, unreacted Fe+ is then titrated by coulometry. The total oxidation number of Cu + Bi is measured in Experiment A, and the oxidation number of Bi is determined in Experiment B. The difference gives the oxidation number of Cu. In Experiment A, a sample of BiSrCaCuOx (FM 760.37 + 15.999x) (containing no yttrium) weighing 102.3 mg was dissolved in 100.0 mL of 1 M HCl containing 2.000 mM CuCl. After reaction with the superconductor, coulometry detected 0.1085 mmol of unreacted Cut in the solution. In Experiment B, 94.6 mg of superconductor were dissolved in 100.0 mL of 1 M HCl containing 1.000 mM FeCl4 HO. After reaction with the superconductor, coulometry detected 0.0577 mmol of unreacted Fe2+. Find the average oxidation numbers of Bi and Cu in the superconductor and the oxygen stoichiometry coefficient, x. oxidation number of Bi: oxidation number of Bi: oxidation number of Cu: X = ^^ MY
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


