Question: We use a simple lattice model to understand why do materials diffuse, in a similar fashion that we study why does energy exchange in class.
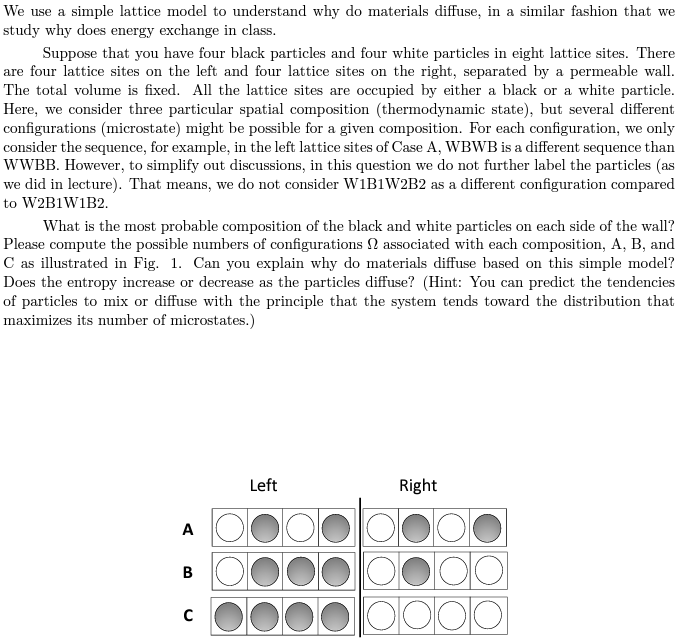
We use a simple lattice model to understand why do materials diffuse, in a similar fashion that we study why does energy exchange in class. Suppose that you have four black particles and four white particles in eight lattice sites. There are four lattice sites on the left and four lattice sites on the right, separated by a permeable wall. The total volume is fixed. All the lattice sites are occupied by either a black or a white particle. Here, we consider three particular spatial composition (thermodynamic state), but several different configurations (microstate) might be possible for a given composition. For each configuration, we only consider the sequence, for example, in the left lattice sites of Case A, WBWB is a different sequence than WWBB. However, to simplify out discussions, in this question we do not further label the particles (as we did in lecture). That means, we do not consider W1B1W2B2 as a different configuration compared to W2B1W1B2. What is the most probable composition of the black and white particles on each side of the wall? Please compute the possible numbers of configurations associated with each composition, A, B, and C as illustrated in Fig. 1. Can you explain why do materials diffuse based on this simple model? Does the entropy increase or decrease as the particles diffuse? (Hint: You can predict the tendencies of particles to mix or diffuse with the principle that the system tends toward the distribution that maximizes its number of microstates.) Left Right A B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


