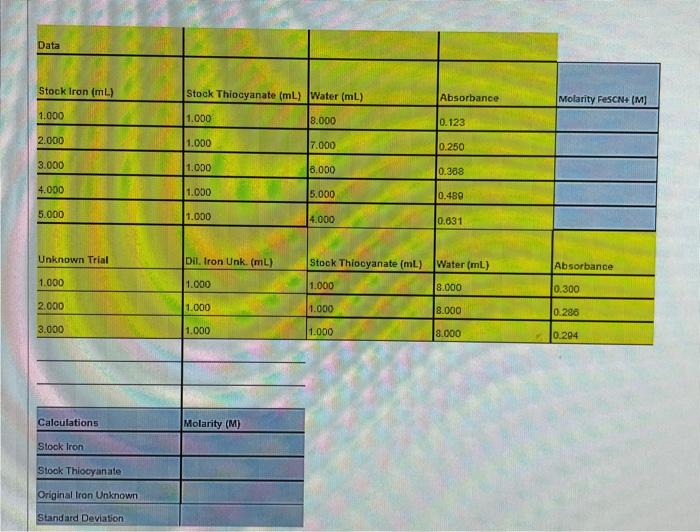
Question: What are the molarities and how do i solve for them? Fill in the blue boxes In preparing a standard calibration curve the following procedure
What are the molarities and how do i solve for them?
Fill in the blue boxes
In preparing a standard calibration curve the following procedure is followed:
A solution of 0.100 M FeCIs in water is provided.
b. 20.00 ml of this 0.100 M FeCls solution is placed in a 500 mL volumetric flask and the solution is diluted to the mark with water (Labelled Stock Iron Solution).
c.
A solution of 0.500 M NaSCN in water is provided
d. 10.00 mL of this 0.500 M NaSCN solution is added to a 250 mL volumetric flask and the solution is diluted to the mark with water (Labelled Stock Thiocyanate Solution).
.
Five trials were undertaken using the following amounts of Stock Iron, Stock Thiocyanate and Water and the Absorbance values of these 5 solutions were collected.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


