Question: Methanol (CH3OH) is produced by reacting CO and H2 according to the equation CO+2H2 CHOH Only 15% of the CO entering the reactor is
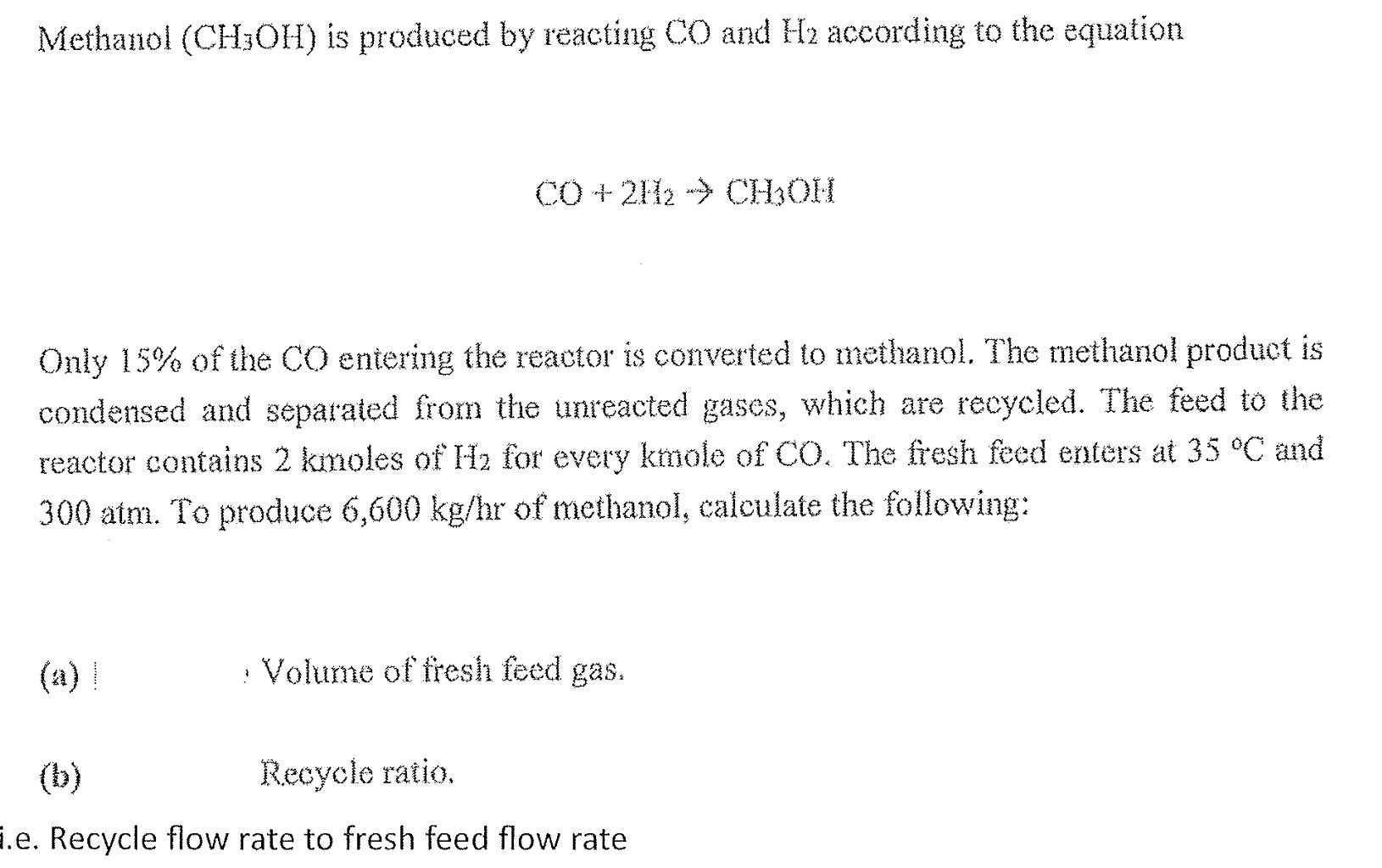
Methanol (CH3OH) is produced by reacting CO and H2 according to the equation CO+2H2 CHOH Only 15% of the CO entering the reactor is converted to methanol. The methanol product is condensed and separated from the unreacted gases, which are recycled. The feed to the reactor contains 2 kmoles of H for every kmole of CO. The fresh feed enters at 35 C and 300 atm. To produce 6,600 kg/hr of methanol, calculate the following: Volume of fresh feed gas. Recycle ratio. i.e. Recycle flow rate to fresh feed flow rate
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Calculating Methanol Production Parameters Given Methanol equation CO 2H2 CH3OH CO conversion 15 Fee... View full answer

Get step-by-step solutions from verified subject matter experts


