Intermolecular forces play vital and varied roles in nature. For example, these forces enable gecko lizards to
Question:
Intermolecular forces play vital and varied roles in nature. For example, these forces enable gecko lizards to climb walls and hang upside down from ceilings, seemingly defying gravity. Intermolecular forces—more specifically, hydrogen bonds—are the reason that DNA molecules, carriers of the genetic code for most living organisms, exist as a double helix. The helical structure of proteins, the molecules that catalyze biochemical reactions occurring in our bodies and regulate metabolic processes, is also the result of hydrogen bonding. We learned about the physical basis of different types of intermolecular forces, such as dipole–dipole, dipole–induced dipole, and instantaneous dipole–induced dipole (dispersion) interactions. We also discussed the relative strengths of these different types of interactions and the percent contributions they make to the attraction between molecules. This problem focuses on doing calculations to verify the claims. For two identical molecules separated from each other by a distance much greater than their own dimensions, the average potential energy of interaction, E, is approximately
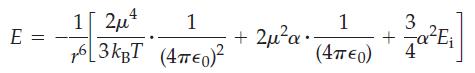
In the equation above, μ is the molecular dipole moment in C m, α is the molecular polarizability in m3, Ei is the first ionization energy of the molecule in J, and r is the center of mass separation in m between the two molecules. In addition, ϵ0 = 8.854 x 10-12 C2J-1 m-1 is the permittivity of vacuum, kB = 1.3807 x 10-23 J K-1 is the Boltzmann constant, and T is the temperature in K. The first term in the equation above represents the dipole–dipole interaction, the second term represents the dipole–induced dipole interaction, and the third term represents the dispersion interaction. Use the equation above and data from the table that follows to answer the questions below. Assume the center of mass separation, r, between molecules is exactly 400 pm and the temperature is 298 K.
(a) For each substance in the table, calculate E, as well as the contributions to E from dipole–dipole, dipole– induced dipole, and dispersion interactions. Express E and the various contributions to E in kJ mol–1.
(b) Use your results from (a) to calculate, for each substance, the percent contribution made by each type of interaction.
(c) What is the range of values of E calculated in (a)? Briefly comment on how these values compare in magnitude with the (covalent) bond energies, D, given in Table 10.3.
(d) Use your results from (a) to prepare three separate graphs of ΔvapH versus –E, one graph for each class of compounds shown in the table (halides, alcohols, and hydrocarbons). What do these graphs illustrate?
(e) The formula for E indicates that the contribution from dipole–dipole interactions decreases as temperature increases. Explain.
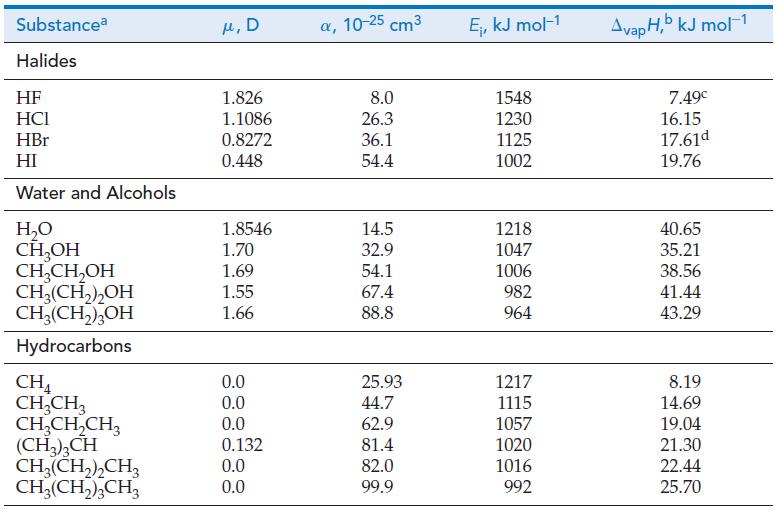
Table 10.3
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




