Question: What does the superscript indicate when associated with a thermodynamic quantity, as in AH, AS, or AG? a. b. It indicates the process is spontaneous
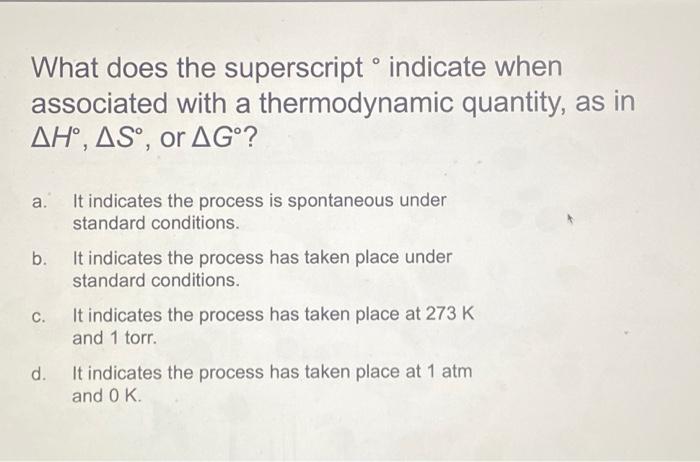
What does the superscript indicate when associated with a thermodynamic quantity, as in H,S, or G? a. It indicates the process is spontaneous under standard conditions. b. It indicates the process has taken place under standard conditions. c. It indicates the process has taken place at 273K and 1 torr. d. It indicates the process has taken place at 1atm and 0K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


