Question: what is the answer? Based upon the mass spectrum shown below, what is true about the molecule? (Any peaks above 46 are impurities.) 100 3-4-5063
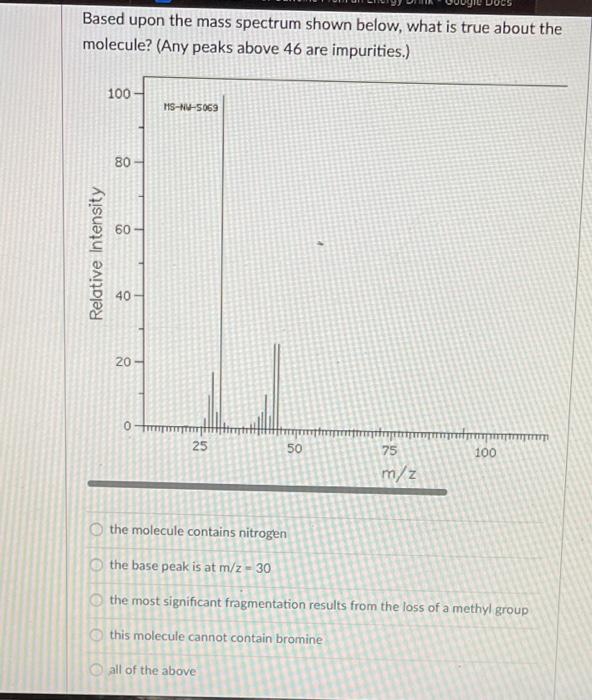
Based upon the mass spectrum shown below, what is true about the molecule? (Any peaks above 46 are impurities.) 100 3-4-5063 80 60 Relative Intensity 40 20 0 25 50 TTTTTTTTT 100 75 m/z the molecule contains nitrogen C the base peak is at m/z - 30 the most significant fragmentation results from the loss of a methyl group this molecule cannot contain bromine all of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


