Question: What is the effect on pH of adding 300.mL of 0.020MKOH(aq) to 1.00L of a solution that contains 0.25MNH3(aq) and 0.25MNH4Cl(aq) ? Data: Kb(NH3)=1.80105 A)
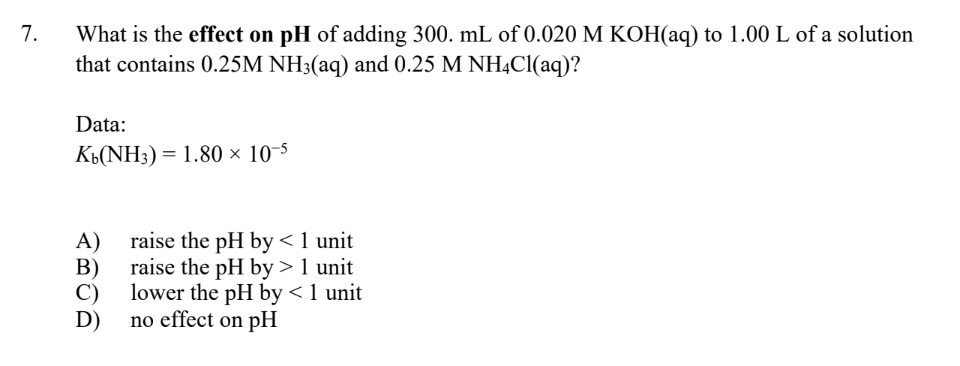
What is the effect on pH of adding 300.mL of 0.020MKOH(aq) to 1.00L of a solution that contains 0.25MNH3(aq) and 0.25MNH4Cl(aq) ? Data: Kb(NH3)=1.80105 A) raise the pH by 1 unit C) lower the pH by
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


