Question: Week 3 Problem Session - Kinetics 1. For the reaction NO(g) + 202(g) NO2(g) + O3(g) the rate = k[NO][O2]. What is the overall order
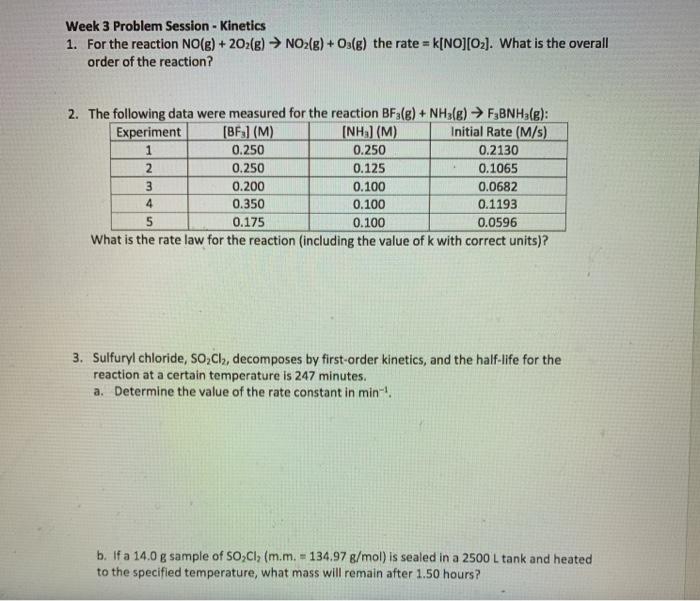
Week 3 Problem Session - Kinetics 1. For the reaction NO(g) + 2O2(g) NO2(g) + Os(g) the rate = k[NO] (O2). What is the overall order of the reaction? 2. The following data were measured for the reaction BFale) + NH3(g) F3BNH3(g): Experiment [BF) (M) [NH) (M) Initial Rate (M/s) 1 0.250 0.250 0.2130 2 0.250 0.125 0.1065 3 0.200 0.100 0.0682 4 0.350 0.100 0.1193 5 0.175 0.100 0.0596 What is the rate law for the reaction (including the value of k with correct units)? 3. Sulfuryl chloride, So,Cly, decomposes by first-order kinetics, and the half-life for the reaction at a certain temperature is 247 minutes. a. Determine the value of the rate constant in min! b. If a 14.0 g sample of So,Cl (m.m. - 134.97 g/mol) is sealed in a 2500 L tank and heated to the specified temperature, what mass will remain after 1.50 hours
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


