Question: What is the net ionic equation for the reaction that occurs between nitrous acid and strontium hydroxide? HNO2(aq)+OH(aq)NO2(aq)+H2O()2HNO2(aq)+Sr(OH)2Sr2(NO2)2(aq)+H2O()2H+(aq)+2NO2(aq)+Sr2+(aq)+2OH(aq)Sr2(NO2)2(aq)+H2O()2H+(aq)+2NO2(aq)+Sr2+(aq)+2OH(aq)Sr2+(aq)+2NO2(aq)+2H2O()H+(aq)+OH(aq)H2O()
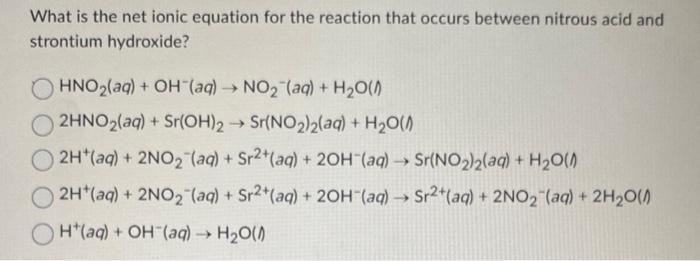
What is the net ionic equation for the reaction that occurs between nitrous acid and strontium hydroxide? HNO2(aq)+OH(aq)NO2(aq)+H2O()2HNO2(aq)+Sr(OH)2Sr2(NO2)2(aq)+H2O()2H+(aq)+2NO2(aq)+Sr2+(aq)+2OH(aq)Sr2(NO2)2(aq)+H2O()2H+(aq)+2NO2(aq)+Sr2+(aq)+2OH(aq)Sr2+(aq)+2NO2(aq)+2H2O()H+(aq)+OH(aq)H2O()
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


